
Wasabi or Japanese horseradish is a plant of the family Brassicaceae, which also includes horseradish and mustard in other genera. The plant is native to Japan and the Russian Far East including Sakhalin, as well as the Korean Peninsula. It grows naturally along stream beds in mountain river valleys in Japan.

Horseradish is a perennial plant of the family Brassicaceae. It is a root vegetable, cultivated and used worldwide as a spice and as a condiment. The species is probably native to Southeastern Europe and Western Asia.

Rapeseed, also known as oilseed rape, is a bright-yellow flowering member of the family Brassicaceae, cultivated mainly for its oil-rich seed, which naturally contains appreciable amounts of erucic acid. The term "canola" denotes a group of rapeseed cultivars that were bred to have very low levels of erucic acid and which are especially prized for use as human and animal food. Rapeseed is the third-largest source of vegetable oil and the second-largest source of protein meal in the world.
Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1ω9. It has the chemical formula: CH3(CH2)7CH=CH(CH2)11CO2H. It is prevalent in wallflower seed and other plants in the family Brassicaceae, with a reported content of 20 to 54% in high erucic acid rapeseed oil and 42% in mustard oil. Erucic acid is also known as cis-13-docosenoic acid and the trans isomer is known as brassidic acid.

In organic chemistry, isothiocyanate is a functional group as found in compounds with the formula R−N=C=S. Isothiocyanates are the more common isomers of thiocyanates, which have the formula R−S−C≡N.

Allyl isothiocyanate (AITC) is a naturally occurring unsaturated isothiocyanate. The colorless oil is responsible for the pungent taste of cruciferous vegetables such as mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels. It is slightly soluble in water, but more soluble in most organic solvents.

Brassica juncea, commonly brown mustard, Chinese mustard, Indian mustard, leaf mustard, Oriental mustard and vegetable mustard, is a species of mustard plant.

Glucoraphanin is a glucosinolate found in broccoli, mustard and other cruciferous vegetables.

Glucosinolates are natural components of many pungent plants such as mustard, cabbage, and horseradish. The pungency of those plants is due to mustard oils produced from glucosinolates when the plant material is chewed, cut, or otherwise damaged. These natural chemicals most likely contribute to plant defence against pests and diseases, and impart a characteristic bitter flavor property to cruciferous vegetables.
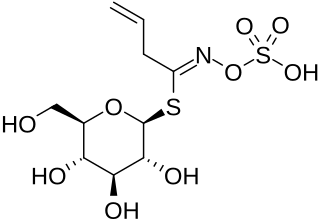
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard. Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil, which is responsible for the pungent taste of mustard and horseradish. Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.
Omega-9 fatty acids are a family of unsaturated fatty acids which have in common a final carbon–carbon double bond in the omega−9 position; that is, the ninth bond from the methyl end of the fatty acid.

Myrosinase is a family of enzymes involved in plant defense against herbivores, specifically the mustard oil bomb. The three-dimensional structure has been elucidated and is available in the PDB.
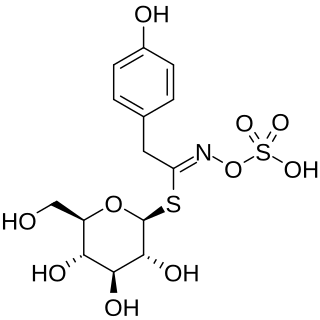
Sinalbin is a glucosinolate found in the seeds of white mustard, Sinapis alba, and in many wild plant species. In contrast to mustard from black mustard seeds which contain sinigrin, mustard from white mustard seeds has only a weakly pungent taste.

Gluconasturtiin or phenethyl glucosinolate is one of the most widely distributed glucosinolates in the cruciferous vegetables, mainly in the roots, and is probably one of the plant compounds responsible for the natural pest-inhibiting properties of growing crucifers, such as cabbage, mustard or rape, in rotation with other crops. This effect of gluconasturtiin is due to its degradation by the plant enzyme myrosinase into phenethyl isothiocyanate, which is toxic to many organisms.

Brevicoryne brassicae, commonly known as the cabbage aphid or cabbage aphis, is a destructive aphid native to Europe that is now found in many other areas of the world. The aphids feed on many varieties of produce, including cabbage, broccoli (especially), Brussels sprouts, cauliflower and many other members of the genus Brassica, but do not feed on plants outside of the family Brassicaceae. The insects entirely avoid plants other than those of Brassicaceae; even though thousands may be eating broccoli near strawberries, the strawberries will be left untouched.

Mustard is a condiment made from the seeds of a mustard plant.

Allyl cyanide is an organic compound with the formula CH2CHCH2CN. Like other small alkyl nitriles, allyl cyanide is colorless and soluble in organic solvents. Allyl cyanide occurs naturally as an antifeedant and is used as a cross-linking agent in some polymers.

Glucotropaeolin or benzyl glucosinolate is a glucosinolate found in cruciferous vegetables, particularly garden cress. Upon enzymatic activity, it is transformed into benzyl isothiocyanate, which contributes to the characteristic flavor of these brassicas.

Rapeseed oil is one of the oldest known vegetable oils. There are both edible and industrial forms produced from rapeseed, the seed of several cultivars of the plant family Brassicaceae. Historically, it was restricted as a food oil due to its content of erucic acid, which in laboratory studies was shown to be damaging to the cardiac muscle of laboratory animals in high quantities and which imparts a bitter taste, and glucosinolates, which made many parts of the plant less nutritious in animal feed. Rapeseed oil from standard cultivars can contain up to 54% erucic acid.


















