Sanofi S.A. is a French multinational pharmaceutical and healthcare company headquartered in Paris, France. The corporation was established in 1973 and merged with Synthélabo in 1999 to form Sanofi-Synthélabo. In 2004, Sanofi-Synthélabo merged with Aventis and renamed to Sanofi-Aventis, which were each the product of several previous mergers. It changed its name back to Sanofi in May 2011. The company is a component of the Euro Stoxx 50 stock market index.

Rosuvastatin, sold under the brand name Crestor among others, is a statin medication, used to prevent cardiovascular disease in those at high risk and treat abnormal lipids. It is recommended to be used together with dietary changes, exercise, and weight loss. It is taken orally.

Off-label use is the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration. Both prescription drugs and over-the-counter drugs (OTCs) can be used in off-label ways, although most studies of off-label use focus on prescription drugs.
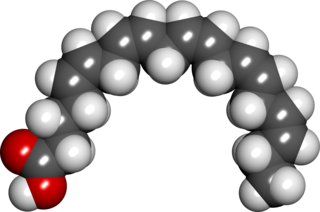
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5(n-3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.
Takeda Oncology is a biopharmaceutical company based in Cambridge, Massachusetts. It is a fully owned subsidiary of Takeda Pharmaceutical.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.

Sodium oxybate, sold under the brand name Xyrem among others, is a medication used to treat symptoms of narcolepsy: sudden muscle weakness and excessive daytime sleepiness. It is used sometimes in France and Italy as an anesthetic given intravenously; it is also approved and used in Italy and in Austria to treat alcohol dependence and alcohol withdrawal syndrome.

Regeneron Pharmaceuticals, Inc. is an American biotechnology company headquartered in Westchester County, New York. The company was founded in 1988. Originally focused on neurotrophic factors and their regenerative capabilities, giving rise to its name, the company then branched out into the study of both cytokine and tyrosine kinase receptors, which gave rise to their first product, which is a VEGF-trap.
Tocilizumab, sold under the brand name Actemra among others, is an immunosuppressive drug, used for the treatment of rheumatoid arthritis, systemic juvenile idiopathic arthritis, a severe form of arthritis in children, and COVID‑19. It is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). Interleukin 6 (IL-6) is a cytokine that plays an important role in immune response and is implicated in the pathogenesis of many diseases, such as autoimmune diseases, multiple myeloma and prostate cancer. Tocilizumab was jointly developed by Osaka University and Chugai, and was licensed in 2003 by Hoffmann-La Roche.

Omega-3-acid ethyl esters are a mixture of ethyl eicosapentaenoic acid and ethyl docosahexaenoic acid, which are ethyl esters of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil. Together with dietary changes, they are used to treat high blood triglycerides which may reduce the risk of pancreatitis. They are generally less preferred than statins, and use is not recommended by NHS Scotland as the evidence does not support a decreased risk of heart disease. Omega-3-acid ethyl esters are taken by mouth.

Ethyl eicosapentaenoic acid, sold under the brand name Vascepa among others, is a medication used to treat dyslipidemia and hypertriglyceridemia. It is used in combination with changes in diet in adults with hypertriglyceridemia ≥ 150 mg/dL. Further, it is often required to be used with a statin.
BioCryst Pharmaceuticals, Inc. is an American pharmaceutical company headquartered in Durham, North Carolina. The company is a late stage biotech company that focuses on oral drugs for rare and serious diseases. BioCryst's antiviral drug peramivir (Rapivab) was approved by FDA in December 2014. It has also been approved in Japan, Korea, and China.

Genta Incorporated was a biopharmaceutical company started in La Jolla, California, which discovered and developed innovative drugs for the treatment of patients with cancer. Founded in 1989 by a highly skilled entrepreneur, the company focused on a novel technology known as antisense, which targets gene products that are associated with the onset and progression of serious diseases. At that time, only Ionis Pharmaceuticals, Inc. was conducting significant research with this technology. Antisense is a short span of oligonucleotides – modified DNA structures ranging from about 12-24 bases that selectively bind to specific RNA. The intent is to block expression of an aberrant protein that contributes to the disease of interest. Genta in-licensed three different antisense molecules that blocked Bcl-2, a fibroblast growth factor (FGF), and the gene c-myb, respectively.

GW Pharmaceuticals Limited is a British pharmaceutics company known for its multiple sclerosis treatment product nabiximols which was the first natural cannabis plant derivative to gain market approval in any country. Another cannabis-based product, Epidiolex, was approved for treatment of epilepsy by the US Food and Drug Administration in 2018. It is a subsidiary of Jazz Pharmaceuticals.
Evolocumab, sold under the brand name Repatha, is a monoclonal antibody that is an immunotherapy medication for the treatment of hyperlipidemia.
Jazz Pharmaceuticals plc is a biopharmaceutical company based in Ireland. It was founded in 2003. One of the company's considerable products is the United States Food and Drug Administration (FDA) approved drug Xyrem, the sodium salt of the naturally occurring neurotransmitter γ-Hydroxybutyric acid (GHB). In 2017, net product sales of Xyrem were $1.187 billion, which represented 74% of the company's total net product sales. In 2019, Jazz was granted FDA-approval to market Sunosi with indications for treating excessive daytime sleepiness (EDS) in narcolepsy as well as obstructive sleep apnea (OSA). In 2022, it was announced that Axsome Therapeutics would be acquiring Sunosi from Jazz Pharmaceuticals
Mesoblast Limited is an Australian regenerative medicine company. It seeks to provide treatments for inflammatory ailments, cardiovascular disease, and back pain. The company is led by Silviu Itescu, who founded the company in 2004.
Omega-3 carboxylic acids (Epanova) is a formerly marketed yet still not an Food And Drug Administration (FDA) approved prescription medication–since taken off market by the manufacturer–used alongside a low fat and low cholesterol diet that lowers high triglyceride (fat) levels in adults with very high levels. This was the third class of fish oil-based drug, after omega-3 acid ethyl esters and ethyl eicosapentaenoic acid (Vascepa), to be approved for use as a drug. The first approval by US Food and Drug Administration was granted 05 May 2014. These fish oil drugs are similar to fish oil dietary supplements, but the ingredients are better controlled and have been tested in clinical trials. Specifically, Epanova contained at least 850 mg omega-3-acid ethyl esters per 1 g capsule.
Relief Therapeutics is a Swiss biopharmaceutical company based in Geneva. The company focuses on developing drugs for serious diseases with few or no existing treatment options. Its lead compound, RLF-100, is a synthetic form of a natural peptide that protects the lung. The company was incorporated as Relief Therapeutics Holdings AG (RFLB.S) and listed on the SIX Swiss Exchange in 2016.
Pronova BioPharma is a Norwegian company. In Denmark it is a bulk manufacturer of omega-3 products with a manufacturing plant in Kalundborg. It was acquired by BASF in 2014.








