
Cytokines are a broad and loose category of small proteins important in cell signaling. Due to their size, cytokines cannot cross the lipid bilayer of cells to enter the cytoplasm and therefore typically exert their functions by interacting with specific cytokine receptors on the target cell surface. Cytokines have been shown to be involved in autocrine, paracrine and endocrine signaling as immunomodulating agents.

Myocarditis, also known as inflammatory cardiomyopathy, is an acquired cardiomyopathy due to inflammation of the heart muscle. Symptoms can include shortness of breath, chest pain, decreased ability to exercise, and an irregular heartbeat. The duration of problems can vary from hours to months. Complications may include heart failure due to dilated cardiomyopathy or cardiac arrest.
In immunology, cytokine release syndrome (CRS) is a form of systemic inflammatory response syndrome (SIRS) that can be triggered by a variety of factors such as infections and certain drugs. It refers to cytokine storm syndromes (CSS) and occurs when large numbers of white blood cells are activated and release inflammatory cytokines, which in turn activate yet more white blood cells. CRS is also an adverse effect of some monoclonal antibody medications, as well as adoptive T-cell therapies. When occurring as a result of a medication, it is also known as an infusion reaction.

An asymptomatic carrier is a person or other organism that has become infected with a pathogen, but shows no signs or symptoms.

In hematology, hemophagocytic lymphohistiocytosis (HLH), also known as haemophagocytic lymphohistiocytosis, and hemophagocytic or haemophagocytic syndrome, is an uncommon hematologic disorder seen more often in children than in adults. It is a life-threatening disease of severe hyperinflammation caused by uncontrolled proliferation of benign lymphocytes and macrophages that secrete high amounts of inflammatory cytokines. It is classified as one of the cytokine storm syndromes. There are inherited and non-inherited (acquired) causes of HLH.

Interleukin-18 (IL-18), also known as interferon-gamma inducing factor is a protein which in humans is encoded by the IL18 gene. The protein encoded by this gene is a proinflammatory cytokine. Many cell types, both hematopoietic cells and non-hematopoietic cells, have the potential to produce IL-18. It was first described in 1989 as a factor that induced interferon-γ (IFN-γ) production in mouse spleen cells. Originally, IL-18 production was recognized in Kupffer cells, liver-resident macrophages. However, IL-18 is constitutively expressed in non-hematopoietic cells, such as intestinal epithelial cells, keratinocytes, and endothelial cells. IL-18 can modulate both innate and adaptive immunity and its dysregulation can cause autoimmune or inflammatory diseases.

Interleukin-29 (IL-29) is a cytokine and it belongs to type III interferons group, also termed interferons λ (IFN-λ). IL-29 plays an important role in the immune response against pathogenes and especially against viruses by mechanisms similar to type I interferons, but targeting primarily cells of epithelial origin and hepatocytes.

Toll-like receptor 7, also known as TLR7, is a protein that in humans is encoded by the TLR7 gene. Orthologs are found in mammals and birds. It is a member of the toll-like receptor (TLR) family and detects single stranded RNA.

Signal transducer and activator of transcription 2 is a protein that in humans is encoded by the STAT2 gene. It is a member of the STAT protein family. This protein is critical to the biological response of type I interferons (IFNs). STAT2 sequence identity between mouse and human is only 68%.
Chronic systemic inflammation (SI) is the result of release of pro-inflammatory cytokines from immune-related cells and the chronic activation of the innate immune system. It can contribute to the development or progression of certain conditions such as cardiovascular disease, cancer, diabetes mellitus, chronic kidney disease, non-alcoholic fatty liver disease, autoimmune and neurodegenerative disorders, and coronary heart disease.
An inflammatory cytokine or proinflammatory cytokine is a type of signaling molecule that is secreted from immune cells like helper T cells (Th) and macrophages, and certain other cell types that promote inflammation. They include interleukin-1 (IL-1), IL-6, IL-12, and IL-18, tumor necrosis factor alpha (TNF-α), interferon gamma (IFNγ), and granulocyte-macrophage colony stimulating factor (GM-CSF) and play an important role in mediating the innate immune response. Inflammatory cytokines are predominantly produced by and involved in the upregulation of inflammatory reactions.
The co-epidemic of tuberculosis (TB) and human immunodeficiency virus (HIV) is one of the major global health challenges in the present time. The World Health Organization (WHO) reports 9.2 million new cases of TB in 2006 of whom 7.7% were HIV-infected. Tuberculosis is the most common contagious infection in HIV-Immunocompromised patients leading to death. These diseases act in combination as HIV drives a decline in immunity while tuberculosis progresses due to defective immune status. This condition becomes more severe in case of multi-drug (MDRTB) and extensively drug resistant TB (XDRTB), which are difficult to treat and contribute to increased mortality. Tuberculosis can occur at any stage of HIV infection. The risk and severity of tuberculosis increases soon after infection with HIV. A study on gold miners of South Africa revealed that the risk of TB was doubled during the first year after HIV seroconversion. Although tuberculosis can be a relatively early manifestation of HIV infection, it is important to note that the risk of tuberculosis progresses as the CD4 cell count decreases along with the progression of HIV infection. The risk of TB generally remains high in HIV-infected patients, remaining above the background risk of the general population even with effective immune reconstitution and high CD4 cell counts with antiretroviral therapy.
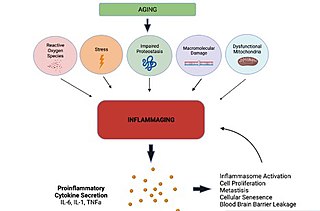
Inflammaging is a chronic, sterile, low-grade inflammation that develops with advanced age, in the absence of overt infection, and may contribute to clinical manifestations of other age-related pathologies. Inflammaging is thought to be caused by a loss of control over systemic inflammation resulting in chronic overstimulation of the innate immune system. Inflammaging is a significant risk factor in mortality and morbidity in aged individuals.

Thirumala-Devi Kanneganti is an immunologist and is the Rose Marie Thomas Endowed Chair, Vice Chair of the Department of Immunology, and Member at St. Jude Children's Research Hospital. She is also Director of the Center of Excellence in Innate Immunity and Inflammation at St. Jude Children's Research Hospital. Her research interests include investigating fundamental mechanisms of innate immunity, including inflammasomes and inflammatory cell death, PANoptosis, in infectious and inflammatory disease and cancer.

Coronavirus disease 2019 (COVID-19) is a contagious disease caused by the virus SARS-CoV-2. The first known case was identified in Wuhan, China, in December 2019. The disease quickly spread worldwide, resulting in the COVID-19 pandemic.

Multisystem inflammatory syndrome in children (MIS-C), or paediatric inflammatory multisystem syndrome, or systemic inflammatory syndrome in COVID-19 (SISCoV), is a rare systemic illness involving persistent fever and extreme inflammation following exposure to SARS-CoV-2, the virus responsible for COVID-19. MIS-C has also been monitored as a potential, rare pediatric adverse event following COVID-19 vaccination. It can rapidly lead to medical emergencies such as insufficient blood flow around the body. Failure of one or more organs can occur. A warning sign is unexplained persistent fever with severe symptoms following exposure to COVID-19. Prompt referral to paediatric specialists is essential, and families need to seek urgent medical assistance. Most affected children will need intensive care. The official MIS-C Awareness Week is in December created by the nonprofit CircumSTANCE. Official awareness items and ribbon can be found on their social media pages or on www.abovecircumstances.org

Dapansutrile (OLT1177) is an inhibitor of the NLRP3 inflammasome.

There is increasing evidence suggesting that COVID-19 causes both acute and chronic neurologicalor psychological symptoms. Caregivers of COVID-19 patients also show a higher than average prevalence of mental health concerns. These symptoms result from multiple different factors.
PANoptosis is an inflammatory cell death pathway. Genetic, molecular, and biochemical studies identified extensive crosstalk among the molecular components across cell death pathways in response to a variety of pathogens and innate immune triggers, leading to the conceptualization of PANoptosis. PANoptosis is defined as a unique innate immune inflammatory cell death pathway driven by caspases and RIPKs and regulated by multi protein PANoptosome complexes. PANoptosis is implicated in driving innate immune responses and inflammation in disease. PANoptosome formation and PANoptosis occur during pathogenic infections, including bacterial, viral, and fungal infections, as well as during inflammatory diseases and can be beneficial in the context of cancer.

Studies have shown that Alzheimer's disease (AD) patients are at an increased risk of morbidity and mortality from SARS-CoV-2, the virus that causes COVID-19. AD is the most common cause of dementia worldwide and is clinically defined by amyloid beta plaques, neurofibrillary tangles, and activation of the brain's immune system. While COVID-19 has been known to more severely impact elderly populations, AD patients have been shown to have a higher rate of SARS-CoV-2 infection compared to cognitively normal patients. The disproportionate risk of COVID-19 in AD patients is thought to arise from an interplay of biological and social factors between the two diseases. Many common biological pathways are shared between COVID-19 and AD, notably those involved in inflammation. Genetic factors that put individuals at risk for AD, such as the APOE4 genotype, are associated with worse outcomes during SARS-CoV-2 infection. Cognitive impairment in AD may prevent patients from following proper public health guidelines, such as masking and social distancing, increasing their risk of infection. Additionally, studies have shown cognitively normal COVID-19 patients are at an increased risk of AD diagnosis following recovery, suggesting that COVID-19 has the potential to cause AD.














