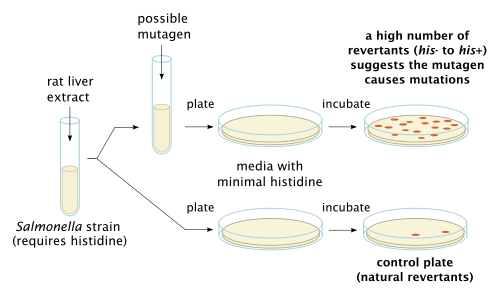
Mutagenesis is a process by which the genetic information of an organism is changed by the production of a mutation. It may occur spontaneously in nature, or as a result of exposure to mutagens. It can also be achieved experimentally using laboratory procedures. A mutagen is a mutation-causing agent, be it chemical or physical, which results in an increased rate of mutations in an organism's genetic code. In nature mutagenesis can lead to cancer and various heritable diseases, and it is also a driving force of evolution. Mutagenesis as a science was developed based on work done by Hermann Muller, Charlotte Auerbach and J. M. Robson in the first half of the 20th century.

In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes.

Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as EtBr, which is also an abbreviation for bromoethane. To avoid confusion, some laboratories have used the abbreviation EthBr for this salt. When exposed to ultraviolet light, it will fluoresce with an orange colour, intensifying almost 20-fold after binding to DNA. Under the name homidium, it has been commonly used since the 1950s in veterinary medicine to treat trypanosomiasis in cattle. The high incidence of antimicrobial resistance makes this treatment impractical in some areas, where the related isometamidium chloride is used instead. Despite its reputation as a mutagen, tests have shown it to have low mutagenicity without metabolic activation.
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.

Phenylbutazone, often referred to as "bute", is a nonsteroidal anti-inflammatory drug (NSAID) for the short-term treatment of pain and fever in animals.
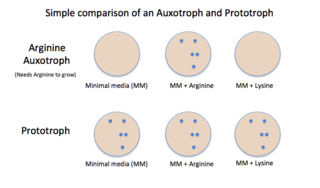
Auxotrophy is the inability of an organism to synthesize a particular organic compound required for its growth. An auxotroph is an organism that displays this characteristic; auxotrophic is the corresponding adjective. Auxotrophy is the opposite of prototrophy, which is characterized by the ability to synthesize all the compounds needed for growth.

Bruce Nathan Ames is an American biochemist. He is a professor of Biochemistry and Molecular Biology Emeritus at the University of California, Berkeley, and was a senior scientist at Children's Hospital Oakland Research Institute (CHORI). He is the inventor of the Ames test, a system for easily and cheaply testing the mutagenicity of compounds.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.

Sudan I is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.

Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.

3,3′,5,5′-Tetramethylbenzidine or TMB is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). TMB is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights.

SYBR Green I (SG) is an asymmetrical cyanine dye used as a nucleic acid stain in molecular biology. The SYBR family of dyes is produced by Molecular Probes Inc., now owned by Thermo Fisher Scientific. SYBR Green I binds to DNA. The resulting DNA-dye-complex best absorbs 497 nanometer blue light and emits green light. The stain preferentially binds to double-stranded DNA, but will stain single-stranded (ss) DNA with lower performance. SYBR Green can also stain RNA with a lower performance than ssDNA.
The S9 fraction is the product of an organ tissue homogenate used in biological assays. The S9 fraction is most frequently used in assays that measure the metabolism of drugs and other xenobiotics. It is defined by the U.S. National Library of Medicine's "IUPAC Glossary of Terms Used in Toxicology" as the "Supernatant fraction obtained from an organ homogenate by centrifuging at 9000 g for 20 minutes in a suitable medium; this fraction contains cytosol and microsomes." The microsomes component of the S9 fraction contain cytochrome P450 isoforms and other enzyme activities. The cytosolic portion contains the major part of the activities of transferases. The S9 fraction is easier to prepare than purified microsomes.
The Environmental Mutagenesis and Genomics Society (EMGS) is a scientific society "for the promotion of critical scientific knowledge and research into the causes and consequences of damage to the genome and epigenome in order to inform and support national and international efforts to ensure a healthy, sustainable environment for future generations."

The SOS chromotest is a biological assay to assess the genotoxic potential of chemical compounds. The test is a colorimetric assay which measures the expression of genes induced by genotoxic agents in Escherichia coli, by means of a fusion with the structural gene for β-galactosidase. The test is performed over a few hours in columns of a 96-well microplate with increasing concentrations of test samples. This test was developed as a practical complement or alternative to the traditional Ames test assay for genotoxicity, which involves growing bacteria on agar plates and comparing natural mutation rates to mutation rates of bacteria exposed to potentially mutagenic compounds or samples. The SOS chromotest is comparable in accuracy and sensitivity to established methods such as the Ames test and is a useful tool to screen genotoxic compounds, which could prove carcinogenic in humans, in order to single out chemicals for further in-depth analysis.
For pharmacology and genetics, the Umu Chromotest, first developed and published by Oda et al., is a biological assay (bioassay) to assess the genotoxic potential of chemical compounds. It is based on the ability of DNA-damaging agents to induce the expression of the umu operon. In connection with the damage inducible (din) genes recA, lexA and umuD, the umuC gene is essentially involved in bacterial mutagenesis through the SOS response.

2-Aminofluorene (2-AF) is a synthetic arylamine. It is a white to tan solid with a melting point of 125-132 °C. 2-AF has only been tested in controlled laboratory settings thus far. There is no indication that it will be tested in industrialized settings. There is evidence that 2-aminofluorene is a carcinogen and an intercalating agent that is extremely dangerous to genomic DNA that potentially can lead to mutation if not death. Furthermore, it has been suggested that 2-aminofluorene can undergo acetylation reactions that causes these reactive species to undergo such reactions in cells. Several experiments have been conducted that have suggested 2-aminofluorene be treated with care and with an overall awareness of the toxicity of this compound.
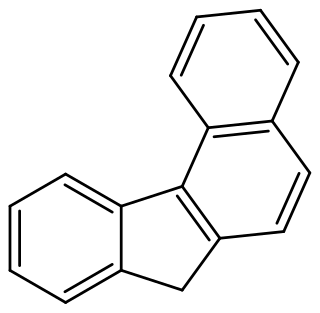
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.

Glycidamide is an organic compound with the formula H2NC(O)C2H3O. It is a colorless, oil. Structurally, it contains adjacent amides and epoxide functional groups. It is a bioactive, potentially toxic or even carcinogenic metabolite of acrylonitrile and acrylamide. It is a chiral molecule.

Francesco De Lorenzo is a physician and politician, member of the Italian Liberal Party. He was born in Naples. He was minister of health (1989–1993) in the Government of Italy. He served in the cabinet of Prime Minister Bettino Craxi (1986–1987), Giulio Andreotti (1989–1992) and Giuliano Amato (1992–1993). He served in the Chamber of Deputies of Italy in Legislature IX (1983–1987), Legislature X (1987–1992) and Legislature XI (1992–1994).