
A goitre, or goiter, is a swelling in the neck resulting from an enlarged thyroid gland. A goitre can be associated with a thyroid that is not functioning properly.

Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidism. Some, however, use the terms interchangeably. Signs and symptoms vary between people and may include irritability, muscle weakness, sleeping problems, a fast heartbeat, heat intolerance, diarrhea, enlargement of the thyroid, hand tremor, and weight loss. Symptoms are typically less severe in the elderly and during pregnancy. An uncommon but life-threatening complication is thyroid storm in which an event such as an infection results in worsening symptoms such as confusion and a high temperature; this often results in death. The opposite is hypothyroidism, when the thyroid gland does not make enough thyroid hormone.

Graves' disease, also known as toxic diffuse goiter, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyroid. Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss. Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves' ophthalmopathy. About 25 to 30% of people with the condition develop eye problems.
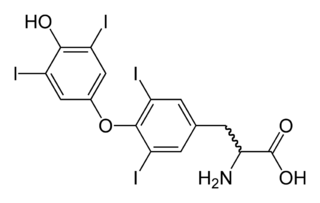
Hypothyroidism is a disorder of the endocrine system in which the thyroid gland does not produce enough thyroid hormones. It can cause a number of symptoms, such as poor ability to tolerate cold, a feeling of tiredness, constipation, slow heart rate, depression, and weight gain. Occasionally there may be swelling of the front part of the neck due to goitre. Untreated cases of hypothyroidism during pregnancy can lead to delays in growth and intellectual development in the baby or congenital iodine deficiency syndrome.
Thyroid-stimulating hormone (also known as thyrotropin, thyrotropic hormone, or abbreviated TSH) is a pituitary hormone that stimulates the thyroid gland to produce thyroxine (T4), and then triiodothyronine (T3) which stimulates the metabolism of almost every tissue in the body. It is a glycoprotein hormone produced by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid.
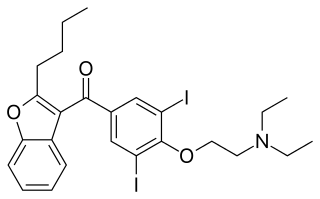
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias. This includes ventricular tachycardia (VT), ventricular fibrillation (VF), and wide complex tachycardia, as well as atrial fibrillation and paroxysmal supraventricular tachycardia. Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin.

Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis and Hashimoto's disease, is an autoimmune disease in which the thyroid gland is gradually destroyed. A slightly broader term is autoimmune thyroiditis, identical other than that it is also used to describe a similar condition without a goitre.
Radiocontrast agents are substances used to enhance the visibility of internal structures in X-ray-based imaging techniques such as computed tomography, projectional radiography, and fluoroscopy. Radiocontrast agents are typically iodine, or more rarely barium sulfate. The contrast agents absorb external X-rays, resulting in decreased exposure on the X-ray detector. This is different from radiopharmaceuticals used in nuclear medicine which emit radiation.

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate.

The Wolff–Chaikoff effect is a presumed reduction in thyroid hormone levels caused by ingestion of a large amount of iodine.
The Jod-Basedow effect is hyperthyroidism following administration of iodine or iodide, either as a dietary supplement, iodinated contrast medical imaging, or as a medication.
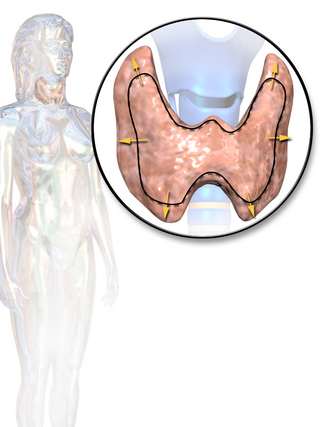
Thyroid disease is a medical condition that affects the function of the thyroid gland. The thyroid gland is located at the front of the neck and produces thyroid hormones that travel through the blood to help regulate many other organs, meaning that it is an endocrine organ. These hormones normally act in the body to regulate energy use, infant development, and childhood development.

Thyroiditis is the inflammation of the thyroid gland. The thyroid gland is located on the front of the neck below the laryngeal prominence, and makes hormones that control metabolism.
Thyroid storm is a rare but severe and life-threatening complication of hyperthyroidism. It occurs when overactive thyroid activity leads to hypermetabolism, the end result being death from cardiac arrest or multiple organ failure.

Subacute thyroiditis refers to a temporal classification of the different forms of thyroiditis based on onset of symptoms. The temporal classification of thyroiditis includes presentation of symptoms in an acute, subacute, or chronic manner. There are also other classification systems for thyroiditis based on factors such as clinical symptoms and underlying etiology.
Thyrotoxic myopathy (TM) is a neuromuscular disorder that develops due to the overproduction of the thyroid hormone thyroxine. Also known as hyperthyroid myopathy, TM is one of many myopathies that lead to muscle weakness and muscle tissue breakdown. Evidence indicates the onset may be caused by hyperthyroidism. Physical symptoms of TM may include muscle weakness, the breakdown of muscle tissue, fatigue, and heat intolerance. Physical acts such as lifting objects and climbing stairs may become increasingly difficult. If untreated, TM can be an extremely debilitating disorder that can, in extreme rare cases, lead to death. If diagnosed and treated properly the effects can be controlled and in most cases reversed leaving no lasting effects.

Thyrotoxic periodic paralysis (TPP) is a rare condition featuring attacks of muscle weakness in the presence of hyperthyroidism. Hypokalemia is usually present during attacks. The condition may be life-threatening if weakness of the breathing muscles leads to respiratory failure, or if the low potassium levels lead to abnormal heart rhythms. If untreated, it is typically recurrent in nature.
Thyroid disease in pregnancy can affect the health of the mother as well as the child before and after delivery. Thyroid disorders are prevalent in women of child-bearing age and for this reason commonly present as a pre-existing disease in pregnancy, or after childbirth. Uncorrected thyroid dysfunction in pregnancy has adverse effects on fetal and maternal well-being. The deleterious effects of thyroid dysfunction can also extend beyond pregnancy and delivery to affect neurointellectual development in the early life of the child. Due to an increase in thyroxine binding globulin, an increase in placental type 3 deioidinase and the placental transfer of maternal thyroxine to the fetus, the demand for thyroid hormones is increased during pregnancy. The necessary increase in thyroid hormone production is facilitated by high human chorionic gonadotropin (hCG) concentrations, which bind the TSH receptor and stimulate the maternal thyroid to increase maternal thyroid hormone concentrations by roughly 50%. If the necessary increase in thyroid function cannot be met, this may cause a previously unnoticed (mild) thyroid disorder to worsen and become evident as gestational thyroid disease. Currently, there is not enough evidence to suggest that screening for thyroid dysfunction is beneficial, especially since treatment thyroid hormone supplementation may come with a risk of overtreatment. After women give birth, about 5% develop postpartum thyroiditis which can occur up to nine months afterwards. This is characterized by a short period of hyperthyroidism followed by a period of hypothyroidism; 20–40% remain permanently hypothyroid.
The signs and symptoms of Graves' disease generally result from the direct and indirect effects of hyperthyroidism; sometimes they are caused by thyroidal conditions, such as Graves' ophthalmopathy, goitre and pretibial myxedema. These clinical manifestations can involve virtually every system in the body. The mechanisms that mediate these effects are not well understood. The severity of the signs and symptoms of hyperthyroidism is related to the duration of the disease, the magnitude of the thyroid hormone excess, and the patient's age. Although the vast majority of patients enjoy significant improvement and remission after proper medical care, health care providers should be aware of variability in the individual response to hyperthyroidism and individual sensitivity to thyroid hormone fluctuations generally. Graves' disease patients can also undergo periods of hypothyroidism, due to the challenges of finding the right dosage of thyroid hormone suppression and/or supplementation. The body's need for thyroid hormone can also change over time, such as in the first months after radioactive iodine treatment (RAI). Thyroid autoimmune diseases can also be volatile: hyperthyroidism can interchange with hypothyroidism and euthyroidism.

The Plummer effect is one of several physiological feedforward mechanisms taking place in follicular cells of the healthy thyroid gland and preventing the development of thyrotoxicosis in situations of extremely high supply with iodine.












