Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidism. Some, however, use the terms interchangeably. Signs and symptoms vary between people and may include irritability, muscle weakness, sleeping problems, a fast heartbeat, heat intolerance, diarrhea, enlargement of the thyroid, hand tremor, and weight loss. Symptoms are typically less severe in the elderly and during pregnancy. An uncommon but life-threatening complication is thyroid storm in which an event such as an infection results in worsening symptoms such as confusion and a high temperature; this often results in death. The opposite is hypothyroidism, when the thyroid gland does not make enough thyroid hormone.

Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.
Radionuclide therapy uses radioactive substances called radiopharmaceuticals to treat medical conditions, particularly cancer. These are introduced into the body by various means and localise to specific locations, organs or tissues depending on their properties and administration routes. This includes anything from a simple compound such as sodium iodide that locates to the thyroid via trapping the iodide ion, to complex biopharmaceuticals such as recombinant antibodies which are attached to radionuclides and seek out specific antigens on cell surfaces.

Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..

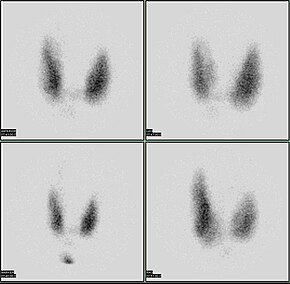
Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by external detectors to form two-dimensional images in a similar process to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images and are therefore classified as separate techniques from scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.

Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. In the third world it is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally.

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate.
Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.

The Wolff–Chaikoff effect is a presumed reduction in thyroid hormone levels caused by ingestion of a large amount of iodine.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
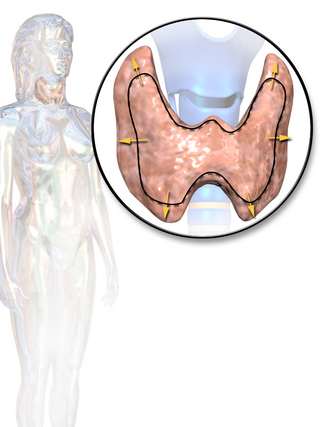
Thyroid disease is a medical condition that affects the function of the thyroid gland. The thyroid gland is located at the front of the neck and produces thyroid hormones that travel through the blood to help regulate many other organs, meaning that it is an endocrine organ. These hormones normally act in the body to regulate energy use, infant development, and childhood development.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV. In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form.

Ioflupane (123I) is the international nonproprietary name (INN) of a cocaine analogue which is a neuro-imaging radiopharmaceutical drug, used in nuclear medicine for the diagnosis of Parkinson's disease and the differential diagnosis of Parkinson's disease over other disorders presenting similar symptoms. During the DaT scan procedure it is injected into a patient and viewed with a gamma camera in order to acquire SPECT images of the brain with particular respect to the striatum, a subcortical region of the basal ganglia. The drug is sold under the brand name Datscan and is manufactured by GE Healthcare, formerly Amersham plc.
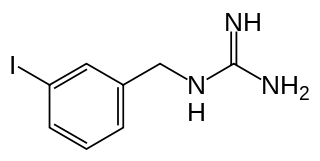
Iobenguane, or MIBG, is an aralkylguanidine analog of the adrenergic neurotransmitter norepinephrine (noradrenaline), typically used as a radiopharmaceutical. It acts as a blocking agent for adrenergic neurons. When radiolabeled, it can be used in nuclear medicinal diagnostic and therapy techniques as well as in neuroendocrine chemotherapy treatments.

Saul Hertz, M.D. was an American physician who devised the medical uses of radioactive iodine. Hertz pioneered the first targeted cancer therapies. Hertz is called the father of the field of theranostics, combining diagnostic imaging with therapy in a single or paired chemical substance(s).
The committed dose in radiological protection is a measure of the stochastic health risk due to an intake of radioactive material into the human body. Stochastic in this context is defined as the probability of cancer induction and genetic damage, due to low levels of radiation. The SI unit of measure is the sievert.

Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents.
Jacob Robbins was an American endocrinologist known for his research on the thyroid gland. He established the "free thyroxine hypothesis", which holds that thyroxine is only active when not bound to protein, and performed long-term research on the incidence of thyroid cancer caused by radiation in survivors of nuclear fallout.