Medical ultrasound includes diagnostic techniques using ultrasound, as well as therapeutic applications of ultrasound. In diagnosis, it is used to create an image of internal body structures such as tendons, muscles, joints, blood vessels, and internal organs, to measure some characteristics or to generate an informative audible sound. The usage of ultrasound to produce visual images for medicine is called medical ultrasonography or simply sonography, or echography. The practice of examining pregnant women using ultrasound is called obstetric ultrasonography, and was an early development of clinical ultrasonography. The machine used is called an ultrasound machine, a sonograph or an echograph. The visual image formed using this technique is called an ultrasonogram, a sonogram or an echogram.

In cardiac physiology, cardiac output (CO), also known as heart output and often denoted by the symbols , , or , is the volumetric flow rate of the heart's pumping output: that is, the volume of blood being pumped by a single ventricle of the heart, per unit time. Cardiac output (CO) is the product of the heart rate (HR), i.e. the number of heartbeats per minute (bpm), and the stroke volume (SV), which is the volume of blood pumped from the left ventricle per beat; thus giving the formula:

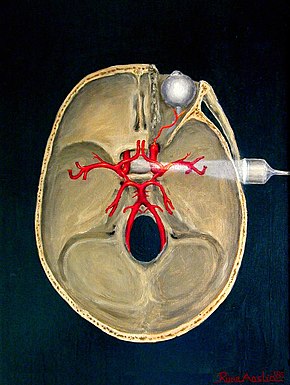
The circle of Willis is a circulatory anastomosis that supplies blood to the brain and surrounding structures in reptiles, birds and mammals, including humans. It is named after Thomas Willis (1621–1675), an English physician.
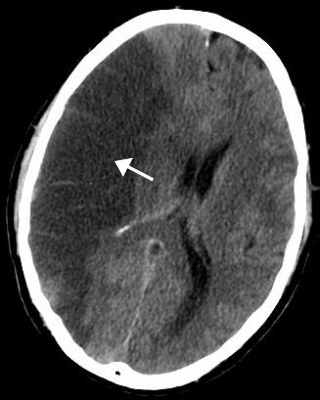
Stroke is a medical condition in which poor blood flow to the brain causes cell death. There are two main types of stroke: ischemic, due to lack of blood flow, and hemorrhagic, due to bleeding. Both cause parts of the brain to stop functioning properly.

Cerebral angiography is a form of angiography which provides images of blood vessels in and around the brain, thereby allowing detection of abnormalities such as arteriovenous malformations and aneurysms. It was pioneered in 1927 by the Portuguese neurologist Egas Moniz at the University of Lisbon, who also helped develop thorotrast for use in the procedure.
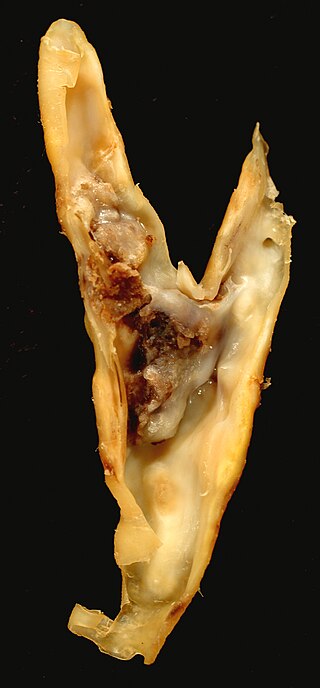
Carotid endarterectomy is a surgical procedure used to reduce the risk of stroke from carotid artery stenosis. In endarterectomy, the surgeon opens the artery and removes the plaque. The plaque forms and thickens the inner layer of the artery, or intima, hence the name of the procedure which simply means removal of part of the internal layers of the artery.

Intraparenchymal hemorrhage (IPH) is one form of intracerebral bleeding in which there is bleeding within brain parenchyma. The other form is intraventricular hemorrhage (IVH).

The cerebral arteries describe three main pairs of arteries and their branches, which perfuse the cerebrum of the brain. The three main arteries are the:
An embolus, is described as a free-floating mass, located inside blood vessels that can travel from one site in the blood stream to another. An embolus can be made up of solid, liquid, or gas. Once these masses get "stuck" in a different blood vessel, it is then known as an "embolism." An embolism can cause ischemia—damage to an organ from lack of oxygen. A paradoxical embolism is a specific type of embolism in which the embolus travels from the right side of the heart to the left side of the heart and lodges itself in a blood vessel known as an artery. Thus, it is termed "paradoxical" because the embolus lands in an artery, rather than a vein.

A watershed stroke is defined as a brain ischemia that is localized to the vulnerable border zones between the tissues supplied by the anterior, posterior and middle cerebral arteries. The actual blood stream blockage/restriction site can be located far away from the infarcts. Watershed locations are those border-zone regions in the brain supplied by the major cerebral arteries where blood supply is decreased. Watershed strokes are a concern because they comprise approximately 10% of all ischemic stroke cases. The watershed zones themselves are particularly susceptible to infarction from global ischemia as the distal nature of the vasculature predisposes these areas to be most sensitive to profound hypoperfusion.
Animal models of ischemic stroke are procedures inducing cerebral ischemia. The aim is the study of basic processes or potential therapeutic interventions in this disease, and the extension of the pathophysiological knowledge on and/or the improvement of medical treatment of human ischemic stroke. Ischemic stroke has a complex pathophysiology involving the interplay of many different cells and tissues such as neurons, glia, endothelium, and the immune system. These events cannot be mimicked satisfactorily in vitro yet. Thus a large portion of stroke research is conducted on animals.

Subclavian steal syndrome (SSS), also called subclavian steal steno-occlusive disease, is a constellation of signs and symptoms that arise from retrograde (reversed) blood flow in the vertebral artery or the internal thoracic artery, due to a proximal stenosis (narrowing) and/or occlusion of the subclavian artery. This flow reversal is called the subclavian steal or subclavian steal phenomenon, regardless of signs/symptoms being present. The arm may be supplied by blood flowing in a retrograde direction down the vertebral artery at the expense of the vertebrobasilar circulation. It is more severe than typical vertebrobasilar insufficiency.

Vertebral artery dissection (VAD) is a flap-like tear of the inner lining of the vertebral artery, which is located in the neck and supplies blood to the brain. After the tear, blood enters the arterial wall and forms a blood clot, thickening the artery wall and often impeding blood flow. The symptoms of vertebral artery dissection include head and neck pain and intermittent or permanent stroke symptoms such as difficulty speaking, impaired coordination, and visual loss. It is usually diagnosed with a contrast-enhanced CT or MRI scan.

The leptomeningeal collateral circulation is a network of small blood vessels in the brain that connects branches of the middle, anterior and posterior cerebral arteries, with variation in its precise anatomy between individuals. During a stroke, leptomeningeal collateral vessels allow limited blood flow when other, larger blood vessels provide inadequate blood supply to a part of the brain.

Carotid ultrasonography is an ultrasound-based diagnostic imaging technique to evaluate structural details of the carotid arteries. Carotid ultrasound is used to diagnose carotid artery stenosis (CAS) and can assess atherosclerotic plaque morphology and characteristics. Carotid duplex and contrast-enhanced ultrasound are two of the most common imaging techniques used to evaluate carotid artery disease.
A silent stroke is a stroke that does not have any outward symptoms associated with stroke, and the patient is typically unaware they have suffered a stroke. Despite not causing identifiable symptoms, a silent stroke still causes damage to the brain and places the patient at increased risk for both transient ischemic attack and major stroke in the future. In a broad study in 1998, more than 11 million people were estimated to have experienced a stroke in the United States. Approximately 770,000 of these strokes were symptomatic and 11 million were first-ever silent MRI infarcts or hemorrhages. Silent strokes typically cause lesions which are detected via the use of neuroimaging such as MRI. The risk of silent stroke increases with age but may also affect younger adults. Women appear to be at increased risk for silent stroke, with hypertension and current cigarette smoking being amongst the predisposing factors.
Increased intracranial pressure (ICP) is one of the major causes of secondary brain ischemia that accompanies a variety of pathological conditions, most notably traumatic brain injury (TBI), strokes, and intracranial hemorrhages. It can cause complications such as vision impairment due to intracranial pressure (VIIP), permanent neurological problems, reversible neurological problems, seizures, stroke, and death. However, aside from a few Level I trauma centers, ICP monitoring is rarely a part of the clinical management of patients with these conditions. The infrequency of ICP can be attributed to the invasive nature of the standard monitoring methods. Additional risks presented to patients can include high costs associated with an ICP sensor's implantation procedure, and the limited access to trained personnel, e.g. a neurosurgeon. Alternative, non-invasive measurement of intracranial pressure, non-invasive methods for estimating ICP have, as a result, been sought.

Doppler ultrasonography is medical ultrasonography that employs the Doppler effect to perform imaging of the movement of tissues and body fluids, and their relative velocity to the probe. By calculating the frequency shift of a particular sample volume, for example, flow in an artery or a jet of blood flow over a heart valve, its speed and direction can be determined and visualized.
Acoustocerebrography (ACG) is a medical test used to diagnose changes and problems in the brain and the central nervous system. It allows for the noninvasive examination of the brain's cellular and molecular structure. It can also be applied as a means to diagnose and monitor intracranial pressure, for example as incorporated into continuous brain monitoring devices. ACG uses molecular acoustics, in audible and ultrasound frequency ranges, to monitor changes. It may use microphones, accelerometers, and multifrequency ultrasonic transducers. It does not use any radiation and is completely free of any side effects. ACG also facilitates blood flow analysis as well as the detection of obstructions in cerebral blood flow (from cerebral embolism) or bleeding (from cerebral hemorrhage).

Functional ultrasound imaging (fUS) is a medical ultrasound imaging technique of detecting or measuring changes in neural activities or metabolism, for example, the loci of brain activity, typically through measuring blood flow or hemodynamic changes. The method can be seen as an extension of Doppler imaging.