Related Research Articles

A microRNA is a small single-stranded non-coding RNA molecule found in plants, animals and some viruses, that functions in RNA silencing and post-transcriptional regulation of gene expression. miRNAs function via base-pairing with complementary sequences within mRNA molecules. As a result, these mRNA molecules are silenced, by one or more of the following processes: (1) cleavage of the mRNA strand into two pieces, (2) destabilization of the mRNA through shortening of its poly(A) tail, and (3) less efficient translation of the mRNA into proteins by ribosomes.
Gene silencing is the regulation of gene expression in a cell to prevent the expression of a certain gene. Gene silencing can occur during either transcription or translation and is often used in research. In particular, methods used to silence genes are being increasingly used to produce therapeutics to combat cancer and other diseases, such as infectious diseases and neurodegenerative disorders.
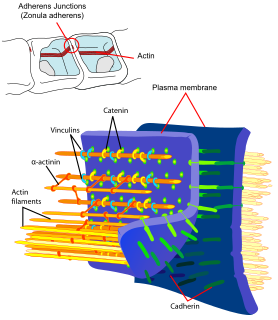
Catenins are a family of proteins found in complexes with cadherin cell adhesion molecules of animal cells. The first two catenins that were identified became known as α-catenin and β-catenin. α-Catenin can bind to β-catenin and can also bind filamentous actin (F-actin). β-Catenin binds directly to the cytoplasmic tail of classical cadherins. Additional catenins such as γ-catenin and δ-catenin have been identified. The name "catenin" was originally selected because it was suspected that catenins might link cadherins to the cytoskeleton.
The epithelial–mesenchymal transition (EMT) is a process by which epithelial cells lose their cell polarity and cell–cell adhesion, and gain migratory and invasive properties to become mesenchymal stem cells; these are multipotent stromal cells that can differentiate into a variety of cell types. EMT is essential for numerous developmental processes including mesoderm formation and neural tube formation. EMT has also been shown to occur in wound healing, in organ fibrosis and in the initiation of metastasis in cancer progression.

The miR-103 microRNA precursor, is a short non-coding RNA gene involved in gene regulation. miR-103 and miR-107 have now been predicted or experimentally confirmed in human.
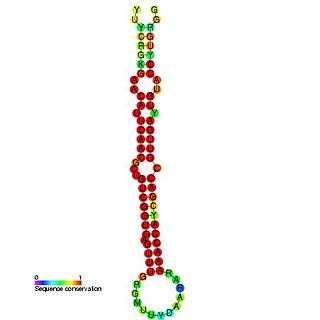
In molecular biology miR-181 microRNA precursor is a small non-coding RNA molecule. MicroRNAs (miRNAs) are transcribed as ~70 nucleotide precursors and subsequently processed by the RNase-III type enzyme Dicer to give a ~22 nucleotide mature product. In this case the mature sequence comes from the 5' arm of the precursor. They target and modulate protein expression by inhibiting translation and / or inducing degradation of target messenger RNAs. This new class of genes has recently been shown to play a central role in malignant transformation. miRNA are downregulated in many tumors and thus appear to function as tumor suppressor genes. The mature products miR-181a, miR-181b, miR-181c or miR-181d are thought to have regulatory roles at posttranscriptional level, through complementarity to target mRNAs. miR-181 which has been predicted or experimentally confirmed in a wide number of vertebrate species as rat, zebrafish, and in the pufferfish.
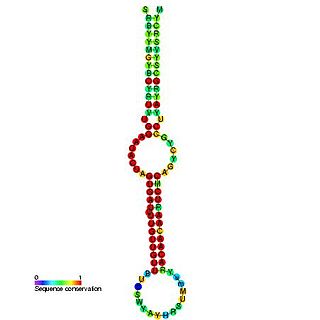
This family represents the microRNA (miRNA) precursor mir-7. This miRNA has been predicted or experimentally confirmed in a wide range of species. miRNAs are transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. In this case the mature sequence comes from the 5' arm of the precursor. The extents of the hairpin precursors are not generally known and are estimated based on hairpin prediction. The involvement of Dicer in miRNA processing suggests a relationship with the phenomenon of RNA interference.

The miR-92 microRNAs are short single stranded non-protein coding RNA fragments initially discovered incorporated into an RNP complex with a proposed role of processing RNA molecules and further RNP assembly. Mir-92 has been mapped to the human genome as part of a larger cluster at chromosome 13q31.3, where it is 22 nucleotides in length but exists in the genome as part of a longer precursor sequence. There is an exact replica of the mir-92 precursor on the X chromosome. MicroRNAs are endogenous triggers of the RNAi pathway which involves several ribonucleic proteins (RNPs) dedicated to repressing mRNA molecules via translation inhibition and/or induction of mRNA cleavage. miRNAs are themselves matured from their long RNA precursors by ribonucleic proteins as part of a 2 step biogenesis mechanism involving RNA polymerase 2.

microRNA 21 also known as hsa-mir-21 or miRNA21 is a mammalian microRNA that is encoded by the MIR21 gene.
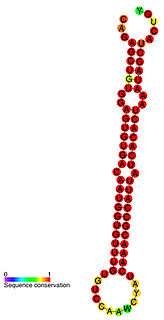
miR-122 is a miRNA that is conserved among vertebrate species. miR-122 is not present in invertebrates, and no close paralogs of miR-122 have been detected. miR-122 is highly expressed in the liver, where it has been implicated as a regulator of fatty-acid metabolism in mouse studies. Reduced miR-122 levels are associated with hepatocellular carcinoma. miR-122 also plays an important positive role in the regulation of hepatitis C virus replication.

In molecular biology mir-126 is a short non-coding RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several pre- and post-transcription mechanisms.
In molecular biology, the miR-200 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by binding and cleaving mRNAs or inhibiting translation. The miR-200 family contains miR-200a, miR-200b, miR-200c, miR-141, and miR-429. There is growing evidence to suggest that miR-200 microRNAs are involved in cancer metastasis.
In molecular biology miR-205 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. They are involved in numerous cellular processes, including development, proliferation, and apoptosis. Currently, it is believed that miRNAs elicit their effect by silencing the expression of target genes.

In molecular biology miR-375 microRNA is a short RNA molecule. MicroRNAs (miRNAs) are small, non-coding RNAs that regulate genes post-transcriptionally by inhibiting translation and/or causing mRNA degradation. miR-375 is found on human chromosome 2 in between the CRYBA2 and CCDC108 genes.
Santaris Pharma A/S was a biopharmaceutical company founded in 2003 in Copenhagen, Denmark. The company also had a branch in San Diego, California that opened in 2009. Created by a merger between Cureon and Pantheco, Santaris developed RNA-targeted medicines using a Locked Nucleic Acid (LNA) Drug Platform and Drug Development Engine.
miR-33 is a family of microRNA precursors, which are processed by the Dicer enzyme to give mature microRNAs. miR-33 is found in several animal species, including humans. In some species there is a single member of this family which gives the mature product mir-33. In humans there are two members of this family called mir-33a and mir-33b, which are located in intronic regions within two protein-coding genes for Sterol regulatory element-binding proteins respectively.
In molecular biology mir-361 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. For example, miR-361-5p might act as a suppressor in triple-negative breast cancer (TNBC) by targeting RQCD1 to inhibit the EGFR/PI3K/Akt signaling pathway.
In molecular biology mir-278 microRNA is a short RNA molecule belonging to a class of molecules referred to as microRNAs. These function to regulate the expression levels of other genes by several mechanisms, primarily binding to their target at its 3'UTR.
Anti-miRNA oligonucleotides have many uses in cellular mechanics. These synthetically designed molecules are used to neutralize microRNA (miRNA) function in cells for desired responses. miRNA are complementary sequences to mRNA that are involved in the cleavage of RNA or the suppression of the translation. By controlling the miRNA that regulate mRNAs in cells, AMOs can be used as further regulation as well as for therapeutic treatment for certain cellular disorders. This regulation can occur through a steric blocking mechanism as well as hybridization to miRNA. These interactions, within the body between miRNA and AMOs, can be for therapeutics in disorders in which over/under expression occurs or aberrations in miRNA lead to coding issues. Some of the miRNA linked disorders that are encountered in the humans include cancers, muscular diseases, autoimmune disorders, and viruses. In order to determine the functionality of certain AMOs, the AMO/miRNA binding expression must be measured against the expressions of the isolated miRNA. The direct detection of differing levels of genetic expression allow the relationship between AMOs and miRNAs to be shown. This can be detected through luciferase activity. Understanding the miRNA sequences involved in these diseases can allow us to use anti miRNA Oligonucleotides to disrupt pathways that lead to the under/over expression of proteins of cells that can cause symptoms for these diseases.
References
- ↑ Yue J (July 2011). "miRNA and vascular cell movement". Adv. Drug Deliv. Rev. 63 (8): 616–22. doi:10.1016/j.addr.2011.01.001. PMC 3129380 . PMID 21241758.
- ↑ Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (December 2005). "Silencing of microRNAs in vivo with 'antagomirs'". Nature. 438 (7068): 685–9. Bibcode:2005Natur.438..685K. doi:10.1038/nature04303. PMID 16258535. S2CID 4414240.
- ↑ Czech MP (March 2006). "MicroRNAs as therapeutic targets". N. Engl. J. Med. 354 (11): 1194–5. doi:10.1056/NEJMcibr060065. PMID 16540623.
- ↑ Ling, Hui; Calin, George A. (2014-01-01), Dellaire, Graham; Berman, Jason. N.; Arceci, Robert J. (eds.), "Chapter 25 - The Role of MicroRNAs and Ultraconserved Non-Coding RNAs in Cancer", Cancer Genomics, Boston: Academic Press, pp. 435–447, doi:10.1016/b978-0-12-396967-5.00025-6, ISBN 978-0-12-396967-5 , retrieved 2022-09-11
- ↑ Raghavendra, Pongali; Pullaiah, Thammineni (2018-01-01), Raghavendra, Pongali; Pullaiah, Thammineni (eds.), "Chapter 2 - RNA-Based Applications in Diagnostic and Therapeutics for Cancer", Advances in Cell and Molecular Diagnostics, Academic Press, pp. 33–55, doi:10.1016/b978-0-12-813679-9.00002-6, ISBN 978-0-12-813679-9 , retrieved 2022-09-11
- ↑ Adam O, Löhfelm B, Thum T, Gupta SK, Puhl SL, Schäfers HJ, Böhm M, Laufs U (September 2012). "Role of miR-21 in the pathogenesis of atrial fibrosis". Basic Res. Cardiol. 107 (5): 278. doi:10.1007/s00395-012-0278-0. PMID 22760500. S2CID 8911862.
- ↑ Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D, Richards T, Selman M, Watkins SC, Pardo A, Ben-Yehudah A, Bouros D, Eickelberg O, Ray P, Benos PV, Kaminski N (July 2010). "Inhibition and role of let-7d in idiopathic pulmonary fibrosis". Am. J. Respir. Crit. Care Med. 182 (2): 220–9. doi:10.1164/rccm.200911-1698OC. PMC 2913236 . PMID 20395557.
- ↑ Kahn CR (December 1978). "Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction". Metab. Clin. Exp. 27 (12 Suppl 2): 1893–902. doi:10.1016/S0026-0495(78)80007-9. PMID 723640.
- ↑ Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M (June 2011). "MicroRNAs 103 and 107 regulate insulin sensitivity". Nature. 474 (7353): 649–53. doi:10.1038/nature10112. PMID 21654750. S2CID 2060531.
- ↑ Young, J. A.; Ting, K. K.; Li, J.; Moller, T.; Dunn, L.; Lu, Y.; Lay, A. J.; Moses, J.; Prado-Lourenco, L.; Khachigian, L. M.; Ng, M.; Gregory, P. A.; Goodall, G. J.; Tsykin, A.; Lichtenstein, I.; Hahn, C. N.; Tran, N.; Shackel, N.; Kench, J. G.; McCaughan, G.; Vadas, M. A.; Gamble, J. R. (5 September 2013). "Regulation of vascular leak and recovery from ischemic injury by general and VE-cadherin-restricted miRNA antagonists of miR-27". Blood. 122 (16): 2911–2919. doi: 10.1182/blood-2012-12-473017 . PMID 24009229.
- ↑ Zhao, Yang; Ting, Kaka; Li, Jia; Cogger, Victoria C; Chen, Jinbiao; Johansson-Percival, Anna; Ngiow, Shin Foong; Holst, Jeff; Grau, Georges E. R.; Goel, Shom; Moller, Thorleif; Dejana, Elisabetta; McCaughan, Geoffrey W; Smyth, Mark J.; Ganss, Ruth; Vadas, Mathew A; Gamble, Jennifer R (27 June 2017). "Targeting vascular endothelial-cadherin in tumor-associated blood vessels promotes T cell-mediated immunotherapy". Cancer Research. 77 (16): 4434–4447. doi: 10.1158/0008-5472.CAN-16-3129 . PMID 28655790.