
(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is an amino acid, abbreviated as Hyp or O, e.g., in Protein Data Bank.

An oligopeptide, often just called peptide, consists of two to twenty amino acids and can include dipeptides, tripeptides, tetrapeptides, and pentapeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied, because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%).

Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 that can be incorporated by special translation mechanisms.
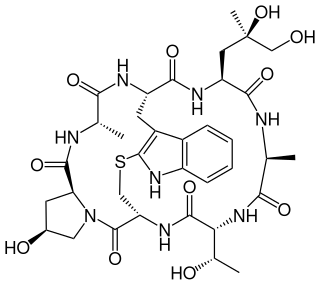
Phalloidin belongs to a class of toxins called phallotoxins, which are found in the death cap mushroom (Amanita phalloides). It is a rigid bicyclic heptapeptide that is lethal after a few days when injected into the bloodstream. The major symptom of phalloidin poisoning is acute hunger due to the destruction of liver cells. It functions by binding and stabilizing filamentous actin (F-actin) and effectively prevents the depolymerization of actin fibers. Due to its tight and selective binding to F-actin, derivatives of phalloidin containing fluorescent tags are used widely in microscopy to visualize F-actin in biomedical research.
Native Chemical Ligation (NCL) is an important extension of the chemical ligation concept for constructing a larger polypeptide chain by the covalent condensation of two or more unprotected peptides segments. Native chemical ligation is the most effective method for synthesizing native or modified proteins of typical size.

Beta-peptides (β-peptides) are peptides derived from β-amino acids, in which the amino group is attached to the β-carbon (i.e. the carbon two atoms away from the carboxylate group). The parent β-amino acid is β-alanine (H2NCH2CH2CO2H), a common natural substance, but most examples feature substituents in place of one or more C-H bonds. β-peptides usually do not occur in nature. β-peptide-based antibiotics are being explored as ways of evading antibiotic resistance. Early studies in this field were published in 1996 by the group of Dieter Seebach and that of Samuel Gellman.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.
Amatoxin is the collective name of a subgroup of at least nine related toxic compounds found in three genera of poisonous mushrooms and one species of the genus Pholiotina. Amatoxins are very potent, as little as half a mushroom cap can cause severe liver injury if swallowed.

Prolyl isomerase is an enzyme found in both prokaryotes and eukaryotes that interconverts the cis and trans isomers of peptide bonds with the amino acid proline. Proline has an unusually conformationally restrained peptide bond due to its cyclic structure with its side chain bonded to its secondary amine nitrogen. Most amino acids have a strong energetic preference for the trans peptide bond conformation due to steric hindrance, but proline's unusual structure stabilizes the cis form so that both isomers are populated under biologically relevant conditions. Proteins with prolyl isomerase activity include cyclophilin, FKBPs, and parvulin, although larger proteins can also contain prolyl isomerase domains.

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

Pseudoproline derivatives are artificially created dipeptides to minimize aggregation during Fmoc solid-phase synthesis of peptides.
The phallotoxins consist of at least seven compounds, all of which are bicyclic heptapeptides, isolated from the death cap mushroom (Amanita phalloides). They differ from the closely related amatoxins by being one residue smaller, both in the final product and the precursor protein.
The discovery of an orally inactive peptide from snake venom established the important role of angiotensin converting enzyme (ACE) inhibitors in regulating blood pressure. This led to the development of captopril, the first ACE inhibitor. When the adverse effects of captopril became apparent new derivates were designed. Then after the discovery of two active sites of ACE: N-domain and C-domain, the development of domain-specific ACE inhibitors began.
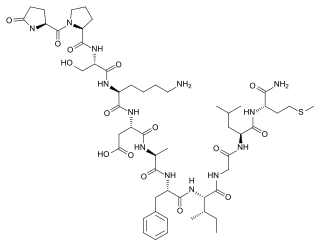
Eledoisin is an undecapeptide of mollusk origin, belonging to the tachykinin family of neuropeptides.
Dipeptidyl peptidase-4 inhibitors are enzyme inhibitors that inhibit the enzyme dipeptidyl peptidase-4 (DPP-4). They are used in the treatment of type 2 diabetes mellitus. Inhibition of the DPP-4 enzyme prolongs and enhances the activity of incretins that play an important role in insulin secretion and blood glucose control regulation. Type 2 diabetes mellitus is a chronic metabolic disease that results from inability of the β-cells in the pancreas to secrete sufficient amounts of insulin to meet the body's needs. Insulin resistance and increased hepatic glucose production can also play a role by increasing the body's demand for insulin. Current treatments, other than insulin supplementation, are sometimes not sufficient to achieve control and may cause undesirable side effects, such as weight gain and hypoglycemia. In recent years, new drugs have been developed, based on continuing research into the mechanism of insulin production and regulation of the metabolism of sugar in the body. The enzyme DPP-4 has been found to play a significant role.
Sunflower trypsin inhibitor (SFTI) is a small, circular peptide produced in sunflower seeds, and is a potent inhibitor of trypsin. It is the smallest known member of the Bowman-Birk family of serine protease inhibitors.
2,5-Diketopiperazine is an organic compound with the formula (NHCH2C(O))2. The compound features a six-membered ring containing two amide groups at opposite positions in the ring. It was first compound containing a peptide bond to be characterized by X-ray crystallography in 1938. It is the parent of a large class of 2,5-Diketopiperazines (2,5-DKPs) with the formula (NHCH2(R)C(O))2 (R = H, CH3, etc.). They are ubiquitous peptide in nature. They are often found in fermentation broths and yeast cultures as well as embedded in larger more complex architectures in a variety of natural products as well as several drugs. In addition, they are often produced as degradation products of polypeptides, especially in processed foods and beverages. They have also been identified in the contents of comets.

Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from Streptomyces bottropensis. It has been shown to inhibit methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE) among other Gram-positive bacteria and mycoplasma. Bottromycin is structurally distinct from both vancomycin, a glycopeptide antibiotic, and methicillin, a beta-lactam antibiotic.
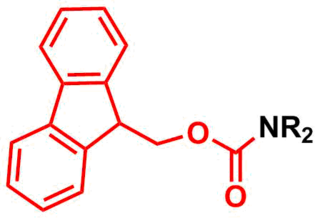
The fluorenylmethoxycarbonyl protecting group (Fmoc) is a base-labile protecting group used in organic synthesis.
Andrimid is an antibiotic natural product that is produced by the marine bacterium Vibrio coralliilyticus. Andrimid is an inhibitor of fatty acid biosynthesis by blocking the carboxyl transfer reaction of acetyl-CoA carboxylase (ACC).













