
Malaria is a mosquito-borne infectious disease that affects humans and other vertebrates. Human malaria causes symptoms that typically include fever, fatigue, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. Symptoms usually begin 10 to 15 days after being bitten by an infected mosquito. If not properly treated, people may have recurrences of the disease months later. In those who have recently survived an infection, reinfection usually causes milder symptoms. This partial resistance disappears over months to years if the person has no continuing exposure to malaria.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.
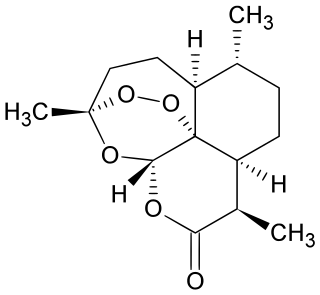
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua, sweet wormwood, a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Artemether is a medication used for the treatment of malaria. The injectable form is specifically used for severe malaria rather than quinine. In adults, it may not be as effective as artesunate. It is given by injection in a muscle. It is also available by mouth in combination with lumefantrine, known as artemether/lumefantrine.

Artesunate (AS) is a medication used to treat malaria. The intravenous form is preferred to quinine for severe malaria. Often it is used as part of combination therapy, such as artesunate plus mefloquine. It is not used for the prevention of malaria. Artesunate can be given by injection into a vein, injection into a muscle, by mouth, and by rectum.

Primaquine is a medication used to treat and prevent malaria and to treat Pneumocystis pneumonia. Specifically it is used for malaria due to Plasmodium vivax and Plasmodium ovale along with other medications and for prevention if other options cannot be used. It is an alternative treatment for Pneumocystis pneumonia together with clindamycin. It is taken by mouth.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.

Plasmodium knowlesi is a parasite that causes malaria in humans and other primates. It is found throughout Southeast Asia, and is the most common cause of human malaria in Malaysia. Like other Plasmodium species, P. knowlesi has a life cycle that requires infection of both a mosquito and a warm-blooded host. While the natural warm-blooded hosts of P. knowlesi are likely various Old World monkeys, humans can be infected by P. knowlesi if they are fed upon by infected mosquitoes. P. knowlesi is a eukaryote in the phylum Apicomplexa, genus Plasmodium, and subgenus Plasmodium. It is most closely related to the human parasite Plasmodium vivax as well as other Plasmodium species that infect non-human primates.
Artemether/lumefantrine, sold under the trade name Coartem among others, is a combination of the two medications artemether and lumefantrine. It is used to treat malaria caused by Plasmodium falciparum that is not treatable with chloroquine. It is not typically used to prevent malaria. It is taken by mouth.

Dihydroartemisinin is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
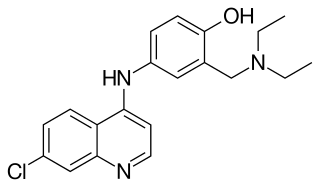
Amodiaquine (ADQ) is a medication used to treat malaria, including Plasmodium falciparum malaria when uncomplicated. It is recommended to be given with artesunate to reduce the risk of resistance. Due to the risk of rare but serious side effects, it is not generally recommended to prevent malaria. Though, the WHO in 2013 recommended use for seasonal preventive in children at high risk in combination with sulfadoxine and pyrimethamine.
Artesunate/amodiaquine, sold under the trade name Camoquin among others, is a medication used for the treatment of malaria. It is a fixed-dose combination of artesunate and amodiaquine. Specifically it recommended for acute uncomplicated Plasmodium falciparum malaria. It is taken by mouth.

The administration of drugs to whole populations irrespective of disease status is referred to as mass drug administration (MDA) or mass dispensing.
Piperaquine is an antiparasitic drug used in combination with dihydroartemisinin to treat malaria. Piperaquine was developed under the Chinese National Malaria Elimination Programme in the 1960s and was adopted throughout China as a replacement for the structurally similar antimalarial drug chloroquine. Due to widespread parasite resistance to piperaquine, the drug fell out of use as a monotherapy, and is instead used as a partner drug for artemisinin combination therapy. Piperaquine kills parasites by disrupting the detoxification of host heme.

Cipargamin is an experimental synthetic antimalarial drug belonging to the spiroindolone class. The compound was developed at the Novartis Institute for Tropical Diseases in Singapore, through a collaboration with the Genomics Institute of the Novartis Research Foundation (GNF), the Biomedical Primate Research Centre and the Swiss Tropical Institute.
Piperaquine/dihydroartemisinin (DHA/PPQ), sold under the brand name Eurartesim among others, is a fixed dose combination medication used in the treatment of malaria. It is a combination of piperaquine and dihydroartemisinin. Specifically it is used for malaria of the P. falciparum and P. vivax types. It is taken by mouth.

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It belongs to the class of the imidazolopiperazines. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.
Artesunate/pyronaridine, sold under the brand name Pyramax, is a fixed-dose combination medication for the treatment of malaria. It can be used for malaria of both the P. falciparum and P. vivax types. It combines artesunate and pyronaridine. It is taken by mouth.
David A. Fidock, is the CS Hamish Young Professor of Microbiology and Immunology and Professor of Medical Sciences at Columbia University Irving Medical Center in Manhattan.

Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains. It contains an organometallic ferrocene ring which is unusual in pharmaceuticals, and while it was first reported in 1997, it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.














