Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry. In humans, the DHFR enzyme is encoded by the DHFR gene. It is found in the q14.1 region of chromosome 5.

Mefloquine, sold under the brand name Lariam among others, is a medication used to prevent or treat malaria. When used for prevention it is typically started before potential exposure and continued for several weeks after potential exposure. It can be used to treat mild or moderate malaria but is not recommended for severe malaria. It is taken by mouth.
Antimalarial medications or simply antimalarials are a type of antiparasitic chemical agent, often naturally derived, that can be used to treat or to prevent malaria, in the latter case, most often aiming at two susceptible target groups, young children and pregnant women. As of 2018, modern treatments, including for severe malaria, continued to depend on therapies deriving historically from quinine and artesunate, both parenteral (injectable) drugs, expanding from there into the many classes of available modern drugs. Incidence and distribution of the disease is expected to remain high, globally, for many years to come; moreover, known antimalarial drugs have repeatedly been observed to elicit resistance in the malaria parasite—including for combination therapies featuring artemisinin, a drug of last resort, where resistance has now been observed in Southeast Asia. As such, the needs for new antimalarial agents and new strategies of treatment remain important priorities in tropical medicine. As well, despite very positive outcomes from many modern treatments, serious side effects can impact some individuals taking standard doses.
Artemisinin and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum. It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery. Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium. Artemisinin is extracted from the plant Artemisia annua a herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.

Proguanil, also known as chlorguanide and chloroguanide, is a medication used to treat and prevent malaria. It is often used together with chloroquine or atovaquone. When used with chloroquine the combination will treat mild chloroquine resistant malaria. It is taken by mouth.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.

Atovaquone, sold under the brand name Mepron, is an antimicrobial medication for the prevention and treatment of Pneumocystis jirovecii pneumonia (PCP).

Pyrimethamine, sold under the brand name Daraprim among others, is a medication used with leucovorin to treat the parasitic diseases toxoplasmosis and cystoisosporiasis. It is also used with dapsone as a second-line option to prevent Pneumocystis jiroveci pneumonia in people with HIV/AIDS. It was previously used for malaria but is no longer recommended due to resistance. Pyrimethamine is taken by mouth.
An apicoplast is a derived non-photosynthetic plastid found in most Apicomplexa, including Toxoplasma gondii, and Plasmodium falciparum and other Plasmodium spp., but not in others such as Cryptosporidium. It originated from algae through secondary endosymbiosis; there is debate as to whether this was a green or red alga. The apicoplast is surrounded by four membranes within the outermost part of the endomembrane system. The apicoplast hosts important metabolic pathways like fatty acid synthesis, isoprenoid precursor synthesis and parts of the heme biosynthetic pathway.
Enoyl-acyl carrier protein reductase, is a key enzyme of the type II fatty acid synthesis (FAS) system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species. In addition, the bacterial ENR sequence and structural organization are distinctly different from those of mammalian fatty acid biosynthesis enzymes.

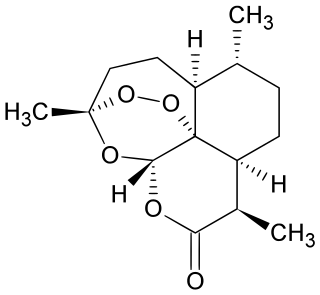
Dihydroartemisinin is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
Malaria prophylaxis is the preventive treatment of malaria. Several malaria vaccines are under development.

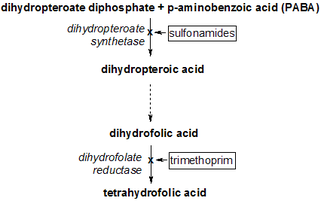
Dihydrofolic acid (conjugate base dihydrofolate) (DHF) is a folic acid (vitamin B9) derivative which is converted to tetrahydrofolic acid by dihydrofolate reductase. Since tetrahydrofolate is needed to make both purines and pyrimidines, which are building blocks of DNA and RNA, dihydrofolate reductase is targeted by various drugs to prevent nucleic acid synthesis.

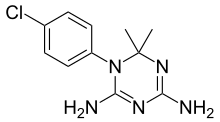
Antifolates are a class of antimetabolite medications that antagonise (that is, block) the actions of folic acid (vitamin B9). Folic acid's primary function in the body is as a cofactor to various methyltransferases involved in serine, methionine, thymidine and purine biosynthesis. Consequently, antifolates inhibit cell division, DNA/RNA synthesis and repair and protein synthesis. Some such as proguanil, pyrimethamine and trimethoprim selectively inhibit folate's actions in microbial organisms such as bacteria, protozoa and fungi. The majority of antifolates work by inhibiting dihydrofolate reductase (DHFR).
A dihydrofolate reductase inhibitor is a molecule that inhibits the function of dihydrofolate reductase, and is a type of antifolate.
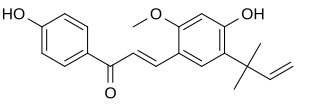
Licochalcone A is a chalconoid, a type of natural phenol. It can be isolated from the root of Glycyrrhiza glabra (liquorice) or Glycyrrhiza inflata. It shows antimalarial, anticancer, antibacterial and antiviral properties in vitro.
Atovaquone/proguanil, sold under the brand name Malarone among others, is a fixed-dose combination medication used to treat and prevent malaria, including chloroquine-resistant malaria. It contains atovaquone and proguanil. It is not recommended for severe or complicated malaria. It is taken by mouth.

Ganaplacide is a drug in development by Novartis for the purpose of treating malaria. It belongs to the class of the imidazolopiperazines. It has shown activity against the Plasmodium falciparum and Plasmodium vivax forms of the malaria parasite.

Ferroquine is a synthetic compound related to chloroquine which acts as an antimalarial, and shows good activity against chloroquine-resistant strains. It contains an organometallic ferrocene ring which is unusual in pharmaceuticals, and while it was first reported in 1997, it has progressed slowly through clinical trials, with results from Phase II trials showing reasonable safety and efficacy, and further trials ongoing.

Gallinamide A is potent and selective inhibitor of the human cysteine protease Cathepsin L1 that was first used as a moderate antimalarial agent. Gallinamide A is produced by marine cyanobacteria from Schizothrix species and Symploca sp. which have also shown to have possible anticancer agent, infectious diseases like leishmaniasis, trypanosomiasis and possible uses in Alzheimer's disease, among others.