
Coke is a grey, hard, and porous coal-based fuel with a high carbon content. It is made by heating coal or oil in the absence of air. Coke is an important industrial product, used mainly in iron ore smelting, but also as a fuel in stoves and forges.

Cremation is a method of final disposition of a dead body through burning.

Incineration is a waste treatment process that involves the combustion of substances contained in waste materials. Industrial plants for waste incineration are commonly referred to as waste-to-energy facilities. Incineration and other high-temperature waste treatment systems are described as "thermal treatment". Incineration of waste materials converts the waste into ash, flue gas and heat. The ash is mostly formed by the inorganic constituents of the waste and may take the form of solid lumps or particulates carried by the flue gas. The flue gases must be cleaned of gaseous and particulate pollutants before they are dispersed into the atmosphere. In some cases, the heat that is generated by incineration can be used to generate electric power.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere.

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

Solid fuel refers to various forms of solid material that can be burnt to release energy, providing heat and light through the process of combustion. Solid fuels can be contrasted with liquid fuels and gaseous fuels. Common examples of solid fuels include wood, charcoal, peat, coal, hexamine fuel tablets, dry dung, wood pellets, corn, wheat, rice, rye, and other grains. Solid fuels are extensively used in rocketry as solid propellants. Solid fuels have been used throughout human history to create fire and solid fuel is still in widespread use throughout the world in the present day.

Wood fuel is a fuel such as firewood, charcoal, chips, sheets, pellets, and sawdust. The particular form used depends upon factors such as source, quantity, quality and application. In many areas, wood is the most easily available form of fuel, requiring no tools in the case of picking up dead wood, or few tools, although as in any industry, specialized tools, such as skidders and hydraulic wood splitters, have been developed to mechanize production. Sawmill waste and construction industry by-products also include various forms of lumber tailings.
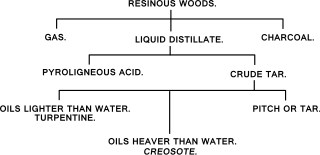
Dry distillation is the heating of solid materials to produce gaseous products. The method may involve pyrolysis or thermolysis, or it may not.

A pyre, also known as a funeral pyre, is a structure, usually made of wood, for burning a body as part of a funeral rite or execution. As a form of cremation, a body is placed upon or under the pyre, which is then set on fire.

A waste-to-energy plant is a waste management facility that combusts wastes to produce electricity. This type of power plant is sometimes called a trash-to-energy, municipal waste incineration, energy recovery, or resource recovery plant.
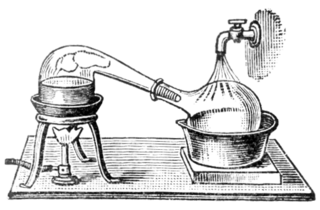
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence of limited amounts of oxygen or other reagents, catalysts, or solvents, such as steam or phenols. It is an application of pyrolysis. The process breaks up or 'cracks' large molecules. Coke, coal gas, gaseous carbon, coal tar, ammonia liquor, and coal oil are examples of commercial products historically produced by the destructive distillation of coal.

Coal pollution mitigation, sometimes labeled as clean coal, is a series of systems and technologies that seek to mitigate health and environmental impact of burning coal for energy. Burning coal releases harmful substances that contribute to air pollution, acid rain, and greenhouse gas emissions. Mitigation includes precombustion approaches, such as cleaning coal, and post combustion approaches, include flue-gas desulfurization, selective catalytic reduction, electrostatic precipitators, and fly ash reduction. These measures aim to reduce coal's impact on human health and the environment.
In analytical chemistry, ashing or ash content determination is the process of mineralization by complete combustion for preconcentration of trace substances prior to a chemical analysis, such as chromatography, or optical analysis, such as spectroscopy.

Smouldering or smoldering is the slow, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel. Many solid materials can sustain a smouldering reaction, including coal, cellulose, wood, cotton, tobacco, cannabis, peat, plant litter, humus, synthetic foams, charring polymers including polyurethane foam and some types of dust. Common examples of smouldering phenomena are the initiation of residential fires on upholstered furniture by weak heat sources, and the persistent combustion of biomass behind the flaming front of wildfires.

Bottom ash is part of the non-combustible residue of combustion in a power plant, boiler, furnace, or incinerator. In an industrial context, it has traditionally referred to coal combustion and comprises traces of combustibles embedded in forming clinkers and sticking to hot side walls of a coal-burning furnace during its operation. The portion of the ash that escapes up the chimney or stack is referred to as fly ash. The clinkers fall by themselves into the bottom hopper of a coal-burning furnace and are cooled. The above portion of the ash is also referred to as bottom ash.

In the context of energy production, biomass is matter from recently living organisms which is used for bioenergy production. Examples include wood, wood residues, energy crops, agricultural residues including straw, and organic waste from industry and households. Wood and wood residues is the largest biomass energy source today. Wood can be used as a fuel directly or processed into pellet fuel or other forms of fuels. Other plants can also be used as fuel, for instance maize, switchgrass, miscanthus and bamboo. The main waste feedstocks are wood waste, agricultural waste, municipal solid waste, and manufacturing waste. Upgrading raw biomass to higher grade fuels can be achieved by different methods, broadly classified as thermal, chemical, or biochemical.

Coal combustion products (CCPs), also called coal combustion wastes (CCWs) or coal combustion residuals (CCRs), are categorized in four groups, each based on physical and chemical forms derived from coal combustion methods and emission controls:

Slash-and-char is an alternative to slash-and-burn that has a lesser effect on the environment. It is the practice of charring the biomass resulting from the slashing, instead of burning it. Due to incomplete combustion (pyrolysis) the resulting residue matter charcoal can be utilized as biochar to improve the soil fertility.

Wood ash is the powdery residue remaining after the combustion of wood, such as burning wood in a fireplace, bonfire, or an industrial power plant. It is largely composed of calcium compounds along with other non-combustible trace elements present in the wood. It has been used for many purposes throughout history.

Charcoal is a lightweight black carbon residue produced by strongly heating wood in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, called charcoal burning, often by forming a charcoal kiln, the heat is supplied by burning part of the starting material itself, with a limited supply of oxygen. The material can also be heated in a closed retort. Modern charcoal briquettes used for outdoor cooking may contain many other additives, e.g. coal.




















