Related Research Articles

The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae, and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors or dysfunction in sorting have been linked to multiple diseases.
The signal recognition particle (SRP) is an abundant, cytosolic, universally conserved ribonucleoprotein that recognizes and targets specific proteins to the endoplasmic reticulum in eukaryotes and the plasma membrane in prokaryotes.
The Coat Protein Complex II, or COPII, is a group of proteins that facilitate the formation of vesicles to transport proteins from the endoplasmic reticulum to the Golgi apparatus or endoplasmic-reticulum–Golgi intermediate compartment. This process is termed anterograde transport, in contrast to the retrograde transport associated with the COPI complex. COPII is assembled in two parts: first an inner layer of Sar1, Sec23, and Sec24 forms; then the inner coat is surrounded by an outer lattice of Sec13 and Sec31.

COPI is a coatomer, a protein complex that coats vesicles transporting proteins from the cis end of the Golgi complex back to the rough endoplasmic reticulum (ER), where they were originally synthesized, and between Golgi compartments. This type of transport is retrograde transport, in contrast to the anterograde transport associated with the COPII protein. The name "COPI" refers to the specific coat protein complex that initiates the budding process on the cis-Golgi membrane. The coat consists of large protein subcomplexes that are made of seven different protein subunits, namely α, β, β', γ, δ, ε and ζ.

Brefeldin A is a lactone antiviral produced by the fungus Penicillium brefeldianum. Brefeldin A inhibits protein transport from the endoplasmic reticulum to the golgi complex indirectly by preventing association of COP-I coat to the Golgi membrane. Brefeldin A was initially isolated with hopes to become an antiviral drug but is now primarily used in research to study protein transport.
The coatomer is a protein complex that coats membrane-bound transport vesicles. Two types of coatomers are known:

Signal recognition particle (SRP) receptor, also called the docking protein, is a dimer composed of 2 different subunits that are associated exclusively with the rough ER in mammalian cells. Its main function is to identify the SRP units. SRP is a molecule that helps the ribosome-mRNA-polypeptide complexes to settle down on the membrane of the endoplasmic reticulum.
In cell signalling, Son of Sevenless (SOS) refers to a set of genes encoding guanine nucleotide exchange factors that act on the Ras subfamily of small GTPases.

Mitofusin-2 is a protein that in humans is encoded by the MFN2 gene. Mitofusins are GTPases embedded in the outer membrane of the mitochondria. In mammals MFN1 and MFN2 are essential for mitochondrial fusion. In addition to the mitofusins, OPA1 regulates inner mitochondrial membrane fusion, and DRP1 is responsible for mitochondrial fission.
Ras-related protein Rab-7a is a protein that in humans is encoded by the RAB7A gene.

KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1, also known as KDELR1, is a protein which in humans is encoded by the KDELR1 gene.
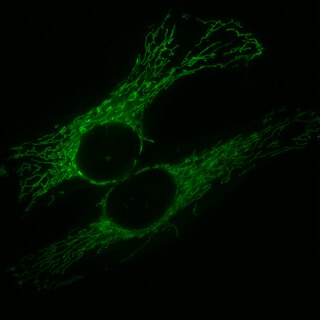
Mitochondrial fission is the process where mitochondria divide or segregate into two separate mitochondrial organelles. Mitochondrial fission is counteracted by the process of mitochondrial fusion, whereby two separate mitochondria can fuse together to form a large one. Mitochondrial fusion in turn can result in elongated mitochondrial networks. Both mitochondrial fission and fusion are balanced in the cell, and mutations interfering with either processes are associated with a variety of diseases. Mitochondria can divide by prokaryotic binary fission and since they require mitochondrial DNA for their function, fission is coordinated with DNA replication. Some of the proteins that are involved in mitochondrial fission have been identified and some of them are associated with mitochondrial diseases. Mitochondrial fission has significant implications in stress response and apoptosis.

Mitochondria are dynamic organelles with the ability to fuse and divide (fission), forming constantly changing tubular networks in most eukaryotic cells. These mitochondrial dynamics, first observed over a hundred years ago are important for the health of the cell, and defects in dynamics lead to genetic disorders. Through fusion, mitochondria can overcome the dangerous consequences of genetic malfunction. The process of mitochondrial fusion involves a variety of proteins that assist the cell throughout the series of events that form this process.
Hereditary sensory and autonomic neuropathy type I or hereditary sensory neuropathy type I is a group of autosomal dominant inherited neurological diseases that affect the peripheral nervous system particularly on the sensory and autonomic functions. The hallmark of the disease is the marked loss of pain and temperature sensation in the distal parts of the lower limbs. The autonomic disturbances, if present, manifest as sweating abnormalities.
DP1/Yop1p is an integral membrane protein family that, along with the reticulons, is responsible for the shape of the tubular endoplasmic reticulum (ER) in yeast and mammalian cells. Furthermore, it is also believed that they might be involved in sheet ER formation.
The endoplasmic reticulum membrane protein complex (EMC) is a putative endoplasmic reticulum-resident membrane protein (co-)chaperone. The EMC is evolutionarily conserved in eukaryotes, and its initial appearance might reach back to the last eukaryotic common ancestor (LECA). Many aspects of mEMC biology and molecular function remain to be studied.

Solute carrier family 25 member 46 is a protein that in humans is encoded by the SLC25A46 gene. This protein is a member of the SLC25 mitochondrial solute carrier family. It is a transmembrane protein located in the mitochondrial outer membrane involved in lipid transfer from the endoplasmic reticulum (ER) to mitochondria. Mutations in this gene result in neuropathy and optic atrophy.

Ras-related protein Rab-2B is a protein that in humans is encoded by the RAB2B gene.
Halperin-Birk syndrome (HLBKS) is a rare autosomal recessive neurodevelopmental disorder caused by a null mutation in the SEC31A gene. Signs and symptoms include intrauterine growth retardation, marked developmental delay, spastic quadriplegia with profound contractures, dysmorphism, and optic nerve atrophy with no eye fixation. Brain MRI demonstrated microcephaly and agenesis of the corpus callosum.
References
- 1 2 3 Rismanchi N, Soderblom C, Stadler J, Zhu PP, Blackstone C (June 2008). "Atlastin GTPases are required for Golgi apparatus and ER morphogenesis". Human Molecular Genetics. 17 (11): 1591–1604. doi:10.1093/hmg/ddn046. PMC 2902292 . PMID 18270207.
- ↑ Zhao G, Zhu PP, Renvoisé B, Maldonado-Báez L, Park SH, Blackstone C (November 2016). "Mammalian knock out cells reveal prominent roles for atlastin GTPases in ER network morphology". Experimental Cell Research. 349 (1): 32–44. doi:10.1016/j.yexcr.2016.09.015. PMID 27669642.
- 1 2 Bian, Xin; Klemm, Robin W.; Liu, Tina Y.; Zhang, Miao; Sun, Sha; Sui, Xuewu; Liu, Xinqi; Rapoport, Tom A.; Hu, Junjie (2011-03-08). "Structures of the atlastin GTPase provide insight into homotypic fusion of endoplasmic reticulum membranes". Proceedings of the National Academy of Sciences. 108 (10): 3976–3981. Bibcode:2011PNAS..108.3976B. doi: 10.1073/pnas.1101643108 . ISSN 0027-8424. PMC 3054032 . PMID 21368113.
- 1 2 Liu, Tina Y.; Bian, Xin; Sun, Sha; Hu, Xiaoyu; Klemm, Robin W.; Prinz, William A.; Rapoport, Tom A.; Hu, Junjie (2012-08-07). "Lipid interaction of the C terminus and association of the transmembrane segments facilitate atlastin-mediated homotypic endoplasmic reticulum fusion". Proceedings of the National Academy of Sciences. 109 (32): E2146-54. doi: 10.1073/pnas.1208385109 . ISSN 0027-8424. PMC 3420179 . PMID 22802620.
- ↑ Byrnes, Laura J; Singh, Avtar; Szeto, Kylan; Benvin, Nicole M; O’Donnell, John P; Zipfel, Warren R; Sondermann, Holger (2013-01-18). "Structural basis for conformational switching and GTP loading of the large G protein atlastin". The EMBO Journal. 32 (3): 369–384. doi:10.1038/emboj.2012.353. ISSN 0261-4189. PMC 3567502 . PMID 23334294.
- 1 2 Byrnes, Laura J.; Sondermann, Holger (2011-02-08). "Structural basis for the nucleotide-dependent dimerization of the large G protein atlastin-1/SPG3A". Proceedings of the National Academy of Sciences. 108 (6): 2216–2221. doi: 10.1073/pnas.1012792108 . ISSN 0027-8424. PMC 3038741 . PMID 21220294.
- ↑ Faust, Joseph E.; Desai, Tanvi; Verma, Avani; Ulengin, Idil; Sun, Tzu-Lin; Moss, Tyler J.; Betancourt-Solis, Miguel A.; Huang, Huey W.; Lee, Tina; McNew, James A. (February 2015). "The Atlastin C-terminal Tail Is an Amphipathic Helix That Perturbs the Bilayer Structure during Endoplasmic Reticulum Homotypic Fusion". Journal of Biological Chemistry. 290 (8): 4772–4783. doi: 10.1074/jbc.M114.601823 . PMC 4335215 . PMID 25555915.
- ↑ Crosby, Daniel; Lee, Tina H. (2022-12-01). Munson, Mary (ed.). "Membrane fusion by Drosophila atlastin does not require GTP hydrolysis". Molecular Biology of the Cell. 33 (14): br23. doi:10.1091/mbc.E22-05-0164. ISSN 1059-1524. PMC 9727788 . PMID 36129776.
- ↑ James Winsor; Ursula Machi; Qixiu Han; David D. Hackney; Tina H. Lee (December 2018). "GTP hydrolysis promotes disassembly of the atlastin crossover dimer during ER fusion". Journal of Cell Biology. 217 (12): 4184–4198. doi:10.1083/jcb.201805039. PMC 6279388 . PMID 30249723.
- ↑ Hu, Xiaoyu; Wu, Fuyun; Sun, Sha; Yu, Wenying; Hu, Junjie (April 2015). "Human atlastin GTPases mediate differentiated fusion of endoplasmic reticulum membranes". Protein & Cell. 6 (4): 307–311. doi:10.1007/s13238-015-0139-3. ISSN 1674-800X. PMC 4383757 . PMID 25773277.
- ↑ Crosby, Daniel; Mikolaj, Melissa R.; Nyenhuis, Sarah B.; Bryce, Samantha; Hinshaw, Jenny E.; Lee, Tina H. (2021-11-24). "Reconstitution of human atlastin fusion activity reveals autoinhibition by the C terminus". Journal of Cell Biology. 221 (2). doi:10.1083/jcb.202107070. ISSN 0021-9525. PMC 8624677 . PMID 34817557.
- 1 2 Park, Seong H.; Zhu, Peng-Peng; Parker, Rell L.; Blackstone, Craig (2010-04-01). "Hereditary spastic paraplegia proteins REEP1, spastin, and atlastin-1 coordinate microtubule interactions with the tubular ER network". Journal of Clinical Investigation. 120 (4): 1097–1110. doi:10.1172/JCI40979. ISSN 0021-9738. PMC 2846052 . PMID 20200447.
- ↑ Jang, Eunhong; Moon, Yeojin; Yoon, So Young; Diaz, Joyce Anne R.; Lee, Miriam; Ko, Naho; Park, Jongseo; Eom, Soo Hyun; Lee, Changwook; Jun, Youngsoo (2023-02-09). "Human atlastins are sufficient to drive the fusion of liposomes with a physiological lipid composition". Journal of Cell Biology. 222 (4). doi:10.1083/jcb.202109090. ISSN 0021-9525. PMC 9949273 . PMID 36757370.
- ↑ Sindhu Wisesa; Yasunori Yamamoto; Toshiaki Sakisaka (November 2019). "TMCC3 localizes at the three-way junctions for the proper tubular network of the endoplasmic reticulum. Biochem". Biochemical Journal. 476 (21): 3241–3260. doi:10.1042/BCJ20190359. hdl: 20.500.14094/D1007853 . PMID 31696206.
- ↑ Monel, Blandine; Rajah, Maaran Michael; Hafirassou, Mohamed Lamine; Sid Ahmed, Samy; Burlaud-Gaillard, Julien; Zhu, Peng-Peng; Nevers, Quentin; Buchrieser, Julian; Porrot, Françoise; Meunier, Cécile; Amraoui, Sonia; Chazal, Maxime; Salles, Audrey; Jouvenet, Nolwenn; Roingeard, Philippe (December 2019). Heise, Mark T. (ed.). "Atlastin Endoplasmic Reticulum-Shaping Proteins Facilitate Zika Virus Replication". Journal of Virology. 93 (23): e01047–19. doi:10.1128/JVI.01047-19. ISSN 0022-538X. PMC 6854498 . PMID 31534046.
- ↑ Kornak, Uwe; Mademan, Inès; Schinke, Marte; Voigt, Martin; Krawitz, Peter; Hecht, Jochen; Barvencik, Florian; Schinke, Thorsten; Gießelmann, Sebastian; Beil, F. Timo; Pou-Serradell, Adolf; Vílchez, Juan J.; Beetz, Christian; Deconinck, Tine; Timmerman, Vincent (March 2014). "Sensory neuropathy with bone destruction due to a mutation in the membrane-shaping atlastin GTPase 3". Brain. 137 (3): 683–692. doi:10.1093/brain/awt357. hdl: 11858/00-001M-0000-0025-78D5-2 . ISSN 1460-2156. PMID 24459106.
- ↑ Leonardis, L.; Auer-Grumbach, M.; Papić, L.; Zidar, J. (July 2012). "The N355K atlastin 1 mutation is associated with hereditary sensory neuropathy and pyramidal tract features". European Journal of Neurology. 19 (7): 992–998. doi:10.1111/j.1468-1331.2012.03665.x. ISSN 1468-1331. PMID 22340599.
- ↑ Fischer, Dirk; Schabhüttl, Maria; Wieland, Thomas; Windhager, Reinhard; Strom, Tim M.; Auer-Grumbach, Michaela (July 2014). "A novel missense mutation confirms ATL3 as a gene for hereditary sensory neuropathy type 1". Brain: A Journal of Neurology. 137 (Pt 7): e286. doi: 10.1093/brain/awu091 . ISSN 1460-2156. PMID 24736309.
- ↑ Mohammadi, Sanaz; Jafari Khamirani, Hossein; Baneshi, Maryam; Kamal, Neda; Manoocheri, Jamal; Saffar, Mahsa; Dianatpour, Mehdi; Tabei, Seyed Mohammad Bagher; Dastgheib, Seyed Alireza (2023-03-01). "A novel nonsense variant in the ATL3 gene is associated with disturbed pain sensitivity, numbness of distal limbs and muscle weakness". Annals of Human Genetics. 87 (4): 147–157. doi:10.1111/ahg.12501. ISSN 1469-1809. PMID 36856139.
- ↑ Krols, Michiel; Detry, Sammy; Asselbergh, Bob; Almeida-Souza, Leonardo; Kremer, Anna; Lippens, Saskia; De Rycke, Riet; De Winter, Vicky; Müller, Franz-Josef; Kurth, Ingo; McMahon, Harvey T.; Savvides, Savvas N.; Timmerman, Vincent; Janssens, Sophie (2018-05-15). "Sensory-Neuropathy-Causing Mutations in ATL3 Cause Aberrant ER Membrane Tethering". Cell Reports. 23 (7): 2026–2038. doi: 10.1016/j.celrep.2018.04.071 . hdl: 1854/LU-8565007 . ISSN 2211-1247. PMID 29768202.
- ↑ Kelly, Carolyn M.; Zeiger, Peter J.; Narayanan, Vinodh; Ramsey, Keri; Sondermann, Holger (January 2022). "A novel insertion mutation in atlastin 1 is associated with spastic quadriplegia, increased membrane tethering, and aberrant conformational switching". The Journal of Biological Chemistry. 298 (1): 101438. doi: 10.1016/j.jbc.2021.101438 . ISSN 1083-351X. PMC 8688574 . PMID 34808209.