
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.

Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized. ESI is different from other ionization processes since it may produce multiple-charged ions, effectively extending the mass range of the analyser to accommodate the kDa-MDa orders of magnitude observed in proteins and their associated polypeptide fragments.
![<span class="mw-page-title-main">Chemical ionization</span> Ionization technique used in mass [[spectroscopy]]](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Chemical_Ionization.png/320px-Chemical_Ionization.png)
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else, must be vapourized before introduction into the source.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.

Thermospray is a soft ionization source by which a solvent flow of liquid sample passes through a very thin heated column to become a spray of fine liquid droplets. As a form of atmospheric pressure ionization in mass spectrometry these droplets are then ionized via a low-current discharge electrode to create a solvent ion plasma. A repeller then directs these charged particles through the skimmer and acceleration region to introduce the aerosolized sample to a mass spectrometer. It is particularly useful in liquid chromatography-mass spectrometry (LC-MS).

Desorption electrospray ionization (DESI) is an ambient ionization technique that can be coupled to mass spectrometry (MS) for chemical analysis of samples at atmospheric conditions. Coupled ionization sources-MS systems are popular in chemical analysis because the individual capabilities of various sources combined with different MS systems allow for chemical determinations of samples. DESI employs a fast-moving charged solvent stream, at an angle relative to the sample surface, to extract analytes from the surfaces and propel the secondary ions toward the mass analyzer. This tandem technique can be used to analyze forensics analyses, pharmaceuticals, plant tissues, fruits, intact biological tissues, enzyme-substrate complexes, metabolites and polymers. Therefore, DESI-MS may be applied in a wide variety of sectors including food and drug administration, pharmaceuticals, environmental monitoring, and biotechnology.
Sample preparation for mass spectrometry is used for the optimization of a sample for analysis in a mass spectrometer (MS). Each ionization method has certain factors that must be considered for that method to be successful, such as volume, concentration, sample phase, and composition of the analyte solution. Quite possibly the most important consideration in sample preparation is knowing what phase the sample must be in for analysis to be successful. In some cases the analyte itself must be purified before entering the ion source. In other situations, the matrix, or everything in the solution surrounding the analyte, is the most important factor to consider and adjust. Often, sample preparation itself for mass spectrometry can be avoided by coupling mass spectrometry to a chromatography method, or some other form of separation before entering the mass spectrometer. In some cases, the analyte itself must be adjusted so that analysis is possible, such as in protein mass spectrometry, where usually the protein of interest is cleaved into peptides before analysis, either by in-gel digestion or by proteolysis in solution.

Laser spray ionization refers to one of several methods for creating ions using a laser interacting with a spray of neutral particles or ablating material to create a plume of charged particles. The ions thus formed can be separated by m/z with mass spectrometry. Laser spray is one of several ion sources that can be coupled with liquid chromatography-mass spectrometry for the detection of larger molecules.

Two-dimensional chromatography is a type of chromatographic technique in which the injected sample is separated by passing through two different separation stages. Two different chromatographic columns are connected in sequence, and the effluent from the first system is transferred onto the second column. Typically the second column has a different separation mechanism, so that bands that are poorly resolved from the first column may be completely separated in the second column. Alternately, the two columns might run at different temperatures. During the second stage of separation the rate at which the separation occurs must be faster than the first stage, since there is still only a single detector. The plane surface is amenable to sequential development in two directions using two different solvents.
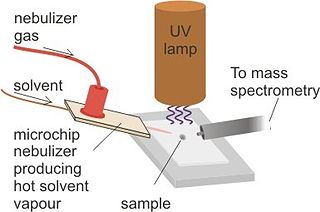
Desorption atmospheric pressure photoionization (DAPPI) is an ambient ionization technique for mass spectrometry that uses hot solvent vapor for desorption in conjunction with photoionization. Ambient Ionization techniques allow for direct analysis of samples without pretreatment. The direct analysis technique, such as DAPPI, eliminates the extraction steps seen in most nontraditional samples. DAPPI can be used to analyze bulkier samples, such as, tablets, powders, resins, plants, and tissues. The first step of this technique utilizes a jet of hot solvent vapor. The hot jet thermally desorbs the sample from a surface. The vaporized sample is then ionized by the vacuum ultraviolet light and consequently sampled into a mass spectrometer. DAPPI can detect a range of both polar and non-polar compounds, but is most sensitive when analyzing neutral or non-polar compounds. This technique also offers a selective and soft ionization for highly conjugated compounds.

Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.

A direct electron ionization liquid chromatography–mass spectrometry interface is a technique for coupling liquid chromatography and mass spectrometry (LC-MS) based on the direct introduction of the liquid effluent into an electron ionization (EI) source. Library searchable mass spectra are generated. Gas-phase EI has many applications for the detection of HPLC amenable compounds showing minimal adverse matrix effects. The direct-EI LC-MS interface provides access to well-characterized electron ionization data for a variety of LC applications and readily interpretable spectra from electronic libraries for environmental, food safety, pharmaceutical, biomedical, and other applications.
Atmospheric pressure laser ionization is an atmospheric pressure ionization method for mass spectrometry (MS). Laser light in the UV range is used to ionize molecules in a resonance-enhanced multiphoton ionization (REMPI) process. It is a selective and sensitive ionization method for aromatic and polyaromatic compounds. Atmospheric photoionization is the latest in development of atmospheric ionization methods.
Ion suppression in LC-MS and LC-MS/MS refers to reduced detector response, or signal:noise as a manifested effect of competition for ionisation efficiency in the ionisation source, between the analyte(s) of interest and other endogenous or exogenous species which have not been removed from the sample matrix during sample preparation. Ion suppression is not strictly a problem unless interfering compounds elute at the same time as the analyte of interest. In cases where ion suppressing species do co-elute with an analyte, the effects on the important analytical parameters including precision, accuracy and limit of detection can be extensive, severely limiting the validity of an assay's results.

Atmospheric pressure photoionization (APPI) is a soft ionization method used in mass spectrometry (MS) usually coupled to liquid chromatography (LC). Molecules are ionized using a vacuum ultraviolet (VUV) light source operating at atmospheric pressure, either by direct absorption followed by electron ejection or through ionization of a dopant molecule that leads to chemical ionization of target molecules. The sample is usually a solvent spray that is vaporized by nebulization and heat. The benefit of APPI is that it ionizes molecules across a broad range of polarity and is particularly useful for ionization of low polarity molecules for which other popular ionization methods such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) are less suitable. It is also less prone to ion suppression and matrix effects compared to ESI and APCI and typically has a wide linear dynamic range. The application of APPI with LC/MS is commonly used for analysis of petroleum compounds, pesticides, steroids, and drug metabolites lacking polar functional groups and is being extensively deployed for ambient ionization particularly for explosives detection in security applications.
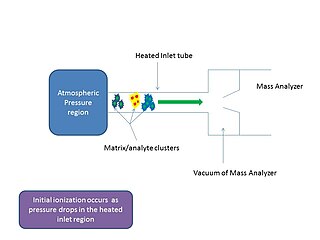
In mass spectrometry, matrix-assisted ionization is a low fragmentation (soft) ionization technique which involves the transfer of particles of the analyte and matrix sample from atmospheric pressure (AP) to the heated inlet tube connecting the AP region to the vacuum of the mass analyzer.

Laser diode thermal desorption (LDTD) is an ionization technique that is coupled to mass spectrometry to analyze samples with atmospheric pressure chemical ionization (APCI). It uses a laser to thermally desorb analytes that are deposited on a stainless steel sheet sample holder, called LazWell. The coupling of LDTD and APCI is considered to be a soft-ionization technique. With LDTD-APCI, it is possible to analyze samples in forensics, pharmaceuticals, environment, food and clinical studies. LDTD is suitable for small molecules between 0 and 1200 Da and some peptides such as cyclosporine.
SCIEX is a manufacturer of mass spectrometry instrumentation used in biomedical and environmental applications. Originally started by scientists from the University of Toronto Institute for Aerospace Studies, it is now part of Danaher Corporation with the SCIEX R&D division still located in Toronto, Canada.






![<span class="mw-page-title-main">Chemical ionization</span> Ionization technique used in mass [[spectroscopy]]](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Chemical_Ionization.png/320px-Chemical_Ionization.png)










