Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.

Epitaxy refers to a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined orientations with respect to the crystalline seed layer. The deposited crystalline film is called an epitaxial film or epitaxial layer. The relative orientation(s) of the epitaxial layer to the seed layer is defined in terms of the orientation of the crystal lattice of each material. For most epitaxial growths, the new layer is usually crystalline and each crystallographic domain of the overlayer must have a well-defined orientation relative to the substrate crystal structure. Epitaxy can involve single-crystal structures, although grain-to-grain epitaxy has been observed in granular films. For most technological applications, single-domain epitaxy, which is the growth of an overlayer crystal with one well-defined orientation with respect to the substrate crystal, is preferred. Epitaxy can also play an important role while growing superlattice structures.
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films is a fundamental step in many applications. A familiar example is the household mirror, which typically has a thin metal coating on the back of a sheet of glass to form a reflective interface. The process of silvering was once commonly used to produce mirrors, while more recently the metal layer is deposited using techniques such as sputtering. Advances in thin film deposition techniques during the 20th century have enabled a wide range of technological breakthroughs in areas such as magnetic recording media, electronic semiconductor devices, integrated passive devices, LEDs, optical coatings, hard coatings on cutting tools, and for both energy generation and storage. It is also being applied to pharmaceuticals, via thin-film drug delivery. A stack of thin films is called a multilayer.

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.
Atomic layer epitaxy (ALE), more generally known as atomic layer deposition (ALD), is a specialized form of thin film growth (epitaxy) that typically deposit alternating monolayers of two elements onto a substrate. The crystal lattice structure achieved is thin, uniform, and aligned with the structure of the substrate. The reactants are brought to the substrate as alternating pulses with "dead" times in between. ALE makes use of the fact that the incoming material is bound strongly until all sites available for chemisorption are occupied. The dead times are used to flush the excess material. It is mostly used in semiconductor fabrication to grow thin films of thickness in the nanometer scale.
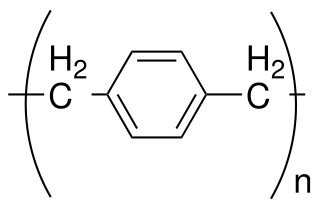
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings –C
6H
4– connected by 1,2-ethanediyl bridges –CH
2–CH
2–. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.

Sputter deposition is a physical vapor deposition (PVD) method of thin film deposition by the phenomenon of sputtering. This involves ejecting material from a "target" that is a source onto a "substrate" such as a silicon wafer. Resputtering is re-emission of the deposited material during the deposition process by ion or atom bombardment. Sputtered atoms ejected from the target have a wide energy distribution, typically up to tens of eV. The sputtered ions can ballistically fly from the target in straight lines and impact energetically on the substrates or vacuum chamber. Alternatively, at higher gas pressures, the ions collide with the gas atoms that act as a moderator and move diffusively, reaching the substrates or vacuum chamber wall and condensing after undergoing a random walk. The entire range from high-energy ballistic impact to low-energy thermalized motion is accessible by changing the background gas pressure. The sputtering gas is often an inert gas such as argon. For efficient momentum transfer, the atomic weight of the sputtering gas should be close to the atomic weight of the target, so for sputtering light elements neon is preferable, while for heavy elements krypton or xenon are used. Reactive gases can also be used to sputter compounds. The compound can be formed on the target surface, in-flight or on the substrate depending on the process parameters. The availability of many parameters that control sputter deposition make it a complex process, but also allow experts a large degree of control over the growth and microstructure of the film.
ASM is a Dutch headquartered multinational corporation that specializes in the design, manufacturing, sales and service of semiconductor wafer processing equipment for the fabrication of semiconductor devices. ASM's products are used by semiconductor manufacturers in front-end wafer processing in their semiconductor fabrication plants. ASM's technologies include atomic layer deposition, epitaxy, chemical vapor deposition and diffusion.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.

A copper indium gallium selenide solar cell is a thin-film solar cell used to convert sunlight into electric power. It is manufactured by depositing a thin layer of copper indium gallium selenide solid solution on glass or plastic backing, along with electrodes on the front and back to collect current. Because the material has a high absorption coefficient and strongly absorbs sunlight, a much thinner film is required than of other semiconductor materials.
Combustion chemical vapor deposition (CCVD) is a chemical process by which thin-film coatings are deposited onto substrates in the open atmosphere.
Chemical Bath Deposition, also called Chemical Solution Deposition and CBD, is a method of thin-film deposition, using an aqueous precursor solution. Chemical Bath Deposition typically forms films using heterogeneous nucleation, to form homogeneous thin films of metal chalcogenides and many less common ionic compounds. Chemical Bath Deposition produces films reliably, using a simple process with little infrastructure, at low temperature (<100˚C), and at low cost. Furthermore, Chemical Bath Deposition can be employed for large-area batch processing or continuous deposition. Films produced by CBD are often used in semiconductors, photovoltaic cells, and supercapacitors, and there is increasing interest in using Chemical Bath Deposition to create nanomaterials.
Eutectic bonding, also referred to as eutectic soldering, describes a wafer bonding technique with an intermediate metal layer that can produce a eutectic system. Those eutectic metals are alloys that transform directly from solid to liquid state, or vice versa from liquid to solid state, at a specific composition and temperature without passing a two-phase equilibrium, i.e. liquid and solid state. The fact that the eutectic temperature can be much lower than the melting temperature of the two or more pure elements can be important in eutectic bonding.
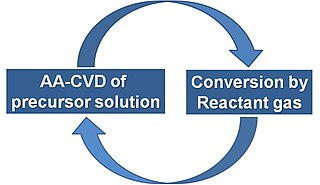
Ion layer gas reaction (ILGAR®) is a non-vacuum, thin-film deposition technique developed and patented by the group of Professor Dr. Christian-Herbert Fischer at the Helmholtz-Zentrum Berlin for materials and energy in Berlin, Germany. It is a sequential and cyclic process that enables the deposition of semiconductor thin films, mainly for photovoltaic applications, specially chalcopyrite absorber layers and buffer layers. The ILGAR technique was awarded as German High Tech Champion 2011 by the Fraunhofer Society.

Tantalum(V) ethoxide is a metalorganic compound with formula Ta2(OC2H5)10, often abbreviated as Ta2(OEt)10. It is a colorless solid that dissolves in some organic solvents but hydrolyzes readily. It is used to prepare films of tantalum(V) oxide.
Valentin Borisovich Aleskovsky was a Soviet and Russian scientist and administrator known for his pioneering research on surface reactions underpinning the thin film deposition technique that years later became known as atomic layer deposition. He was the rector of Leningrad Technological Institute (1962-75) and of Leningrad State University (1975-1986).
Two dimensional hexagonal boron nitride is a material of comparable structure to graphene with potential applications in e.g. photonics., fuel cells and as a substrate for two-dimensional heterostructures. 2D h-BN is isostructural to graphene, but where graphene is conductive, 2D h-BN is a wide-gap insulator.
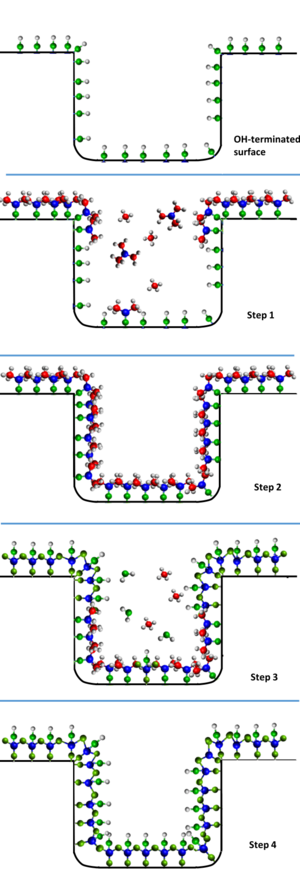
Molecular layer deposition (MLD) is a vapour phase thin film deposition technique based on self-limiting surface reactions carried out in a sequential manner. Essentially, MLD resembles the well established technique of atomic layer deposition (ALD) but, whereas ALD is limited to exclusively inorganic coatings, the precursor chemistry in MLD can use small, bifunctional organic molecules as well. This enables, as well as the growth of organic layers in a process similar to polymerization, the linking of both types of building blocks together in a controlled way to build up organic-inorganic hybrid materials.
Jeffrey Elam is a Distinguished Fellow, Senior Chemist and Group Leader in the Applied Materials Division at the U.S. Department of Energy's Argonne National Laboratory. He leads Argonne's atomic layer deposition (ALD) research program, where he directs research and development and commercialization of thin film coating technologies for energy applications.
Derived from atomic layer deposition (ALD), sequential infiltration synthesis (SIS) is a technique in which a polymer is infused with inorganic material using sequential, self-limiting exposures to gaseous precursors, allowing for the precise control over the composition, structure and properties of materials. This synthesis involves metal-organic vapor-phase precursors and co-reactants dissolving and diffusing into polymers, interacting with the polymer functional groups via reversible complex formation and/or irreversible chemical reactions yielding desired composite materials, which may be nanostructured. The metal-organic precursor (A) and co-react vapor (B) are supplied in an alternating, ABAB… sequence. Following SIS, the organic phase can be removed thermally or chemically to leave only the inorganic components behind. The precise control over the infiltration and synthesis via SIS allows the creation of materials with tailored properties like composition, mechanics, stoichiometry, porosity, conductivity, refractive index, and chemical functionality on the nanoscale. This empowers SIS to have a wide range of applications from electronics to energy storage to catalysis. SIS is sometimes referred to as "multiple pulsed vapor-phase infiltration" (MPI), "vapor phase infiltration" (VPI)” or "sequential vapor infiltration" (SVI)".