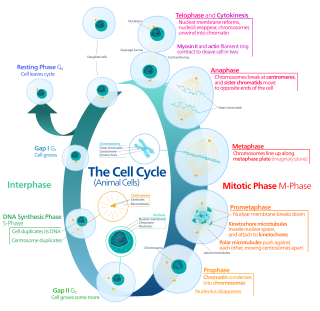
Mitosis is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis is an equational division which gives rise to genetically identical cells in which the total number of chromosomes is maintained. Mitosis is preceded by the S phase of interphase and is followed by telophase and cytokinesis, which divide the cytoplasm, organelles, and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis altogether define the mitotic phase of a cell cycle—the division of the mother cell into two daughter cells genetically identical to each other.

Cell division is the process by which a parent cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukaryotes, there are two distinct types of cell division: a vegetative division (mitosis), producing daughter cells genetically identical to the parent cell, and a cell division that produces haploid gametes for sexual reproduction (meiosis), reducing the number of chromosomes from two of each type in the diploid parent cell to one of each type in the daughter cells. Mitosis is a part of the cell cycle, in which, replicated chromosomes are separated into two new nuclei. Cell division gives rise to genetically identical cells in which the total number of chromosomes is maintained. In general, mitosis is preceded by the S stage of interphase and is followed by telophase and cytokinesis; which divides the cytoplasm, organelles, and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis all together define the M phase of an animal cell cycle—the division of the mother cell into two genetically identical daughter cells. To ensure proper progression through the cell cycle, DNA damage is detected and repaired at various checkpoints throughout the cycle. These checkpoints can halt progression through the cell cycle by inhibiting certain cyclin-CDK complexes. Meiosis undergoes two divisions resulting in four haploid daughter cells. Homologous chromosomes are separated in the first division of meiosis, such that each daughter cell has one copy of each chromosome. These chromosomes have already been replicated and have two sister chromatids which are then separated during the second division of meiosis. Both of these cell division cycles are used in the process of sexual reproduction at some point in their life cycle. Both are believed to be present in the last eukaryotic common ancestor.
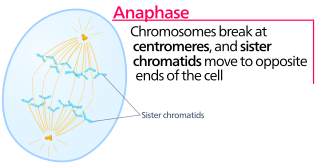
Anaphase is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes are moved to opposite poles of the cell. Chromosomes also reach their overall maximum condensation in late anaphase, to help chromosome segregation and the re-formation of the nucleus.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.

Telophase is the final stage in both meiosis and mitosis in a eukaryotic cell. During telophase, the effects of prophase and prometaphase are reversed. As chromosomes reach the cell poles, a nuclear envelope is re-assembled around each set of chromatids, the nucleoli reappear, and chromosomes begin to decondense back into the expanded chromatin that is present during interphase. The mitotic spindle is disassembled and remaining spindle microtubules are depolymerized. Telophase accounts for approximately 2% of the cell cycle's duration.

The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during metaphase of mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles. Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together.

A kinetochore is a disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart. The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis. The term kinetochore was first used in a footnote in a 1934 Cytology book by Lester W. Sharp and commonly accepted in 1936. Sharp's footnote reads: "The convenient term kinetochore has been suggested to the author by J. A. Moore", likely referring to John Alexander Moore who had joined Columbia University as a freshman in 1932.

Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.
Polo-like kinases (Plks) are regulatory serine/threonine kinases of the cell cycle involved in mitotic entry, mitotic exit, spindle formation, cytokinesis, and meiosis. Only one Plk is found in the genomes of the fly Drosophila melanogaster (Polo), budding yeast (Cdc5) and fission yeast (Plo1). Vertebrates and other animals, however, have many Plk family members including Plk1, Plk2/Snk, Plk3/Prk/FnK, Plk4/Sak and Plk5. Of the vertebrate Plk family members, the mammalian Plk1 has been most extensively studied. During mitosis and cytokinesis, Plks associate with several structures including the centrosome, kinetochores, and the central spindle.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Mitotic checkpoint serine/threonine-protein kinase BUB1 also known as BUB1 is an enzyme that in humans is encoded by the BUB1 gene.

Mitotic checkpoint serine/threonine-protein kinase BUB1 beta is an enzyme that in humans is encoded by the BUB1B gene. Also known as BubR1, this protein is recognized for its mitotic roles in the spindle assembly checkpoint (SAC) and kinetochore-microtubule interactions that facilitate chromosome migration and alignment. BubR1 promotes mitotic fidelity and protects against aneuploidy by ensuring proper chromosome segregation between daughter cells. BubR1 is proposed to prevent tumorigenesis.

Centromere protein A, also known as CENPA, is a protein which in humans is encoded by the CENPA gene. CENPA is a histone H3 variant which is the critical factor determining the kinetochore position(s) on each chromosome in most eukaryotes including humans.

Centromere protein F is a protein that in humans is encoded by the CENPF gene. It is involved in chromosome segregation during cell division. It also has a role in the orientation of microtubules to form cellular cilia.

Inner centromere protein is a protein that in humans is encoded by the INCENP gene. It is a regulatory protein in the chromosome passenger complex (CPC). It is involved in regulation of the catalytic proteins Aurora B and Aurora C. It acts in association with two other proteins - Survivin and Borealin. These proteins form a tight three-helical bundle. The N-terminal domain of INCENP is the domain involved in formation of this three-helical bundle while its C-terminal domain is responsible for the interaction with Aurora B.

Centromere-associated protein E is a protein that in humans is encoded by the CENPE gene.

Kinesin-like protein KIF2C is a protein that in humans is encoded by the KIF2C gene.

Aurora kinase C, also Serine/threonine-protein kinase 13 is an enzyme that in humans is encoded by the AURKC gene.
Syntelic attachment occurs when both sister chromosomes are attached to a single spindle pole.

Tim J. Yen is an American molecular biologist and cancer biologist. Yen held the rank of Professor and in 2023, became Emeritus at Fox Chase Cancer Center in Philadelphia, Pennsylvania. Yen is known for pioneering work in the field of mitosis.