Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products ; this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the following applications:
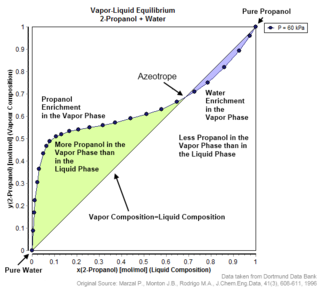
An azeotrope or a constant heating point mixture is a mixture of two or more components in fluidic states whose proportions cannot be altered or changed by simple distillation. This happens because when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Azeotropic mixture behavior is important for fluid separation processes.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.

A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale. Stills have been used to produce perfume and medicine, water for injection (WFI) for pharmaceutical use, generally to separate and purify different chemicals, and to produce distilled beverages containing ethanol.

A pot still is a type of distillation apparatus or still used to distill liquors such as whisky or brandy. In modern (post-1850s) practice, they are not used to produce rectified spirit, because they do not separate congeners from ethanol as effectively as other distillation methods. Pot stills operate on a batch distillation basis. Traditionally constructed from copper, pot stills are made in a range of shapes and sizes depending on the quantity and style of spirit desired.

A column still, also called a continuous still, patent still or Coffey still is a variety of still consisting of two columns. Column stills can produce rectified spirit.
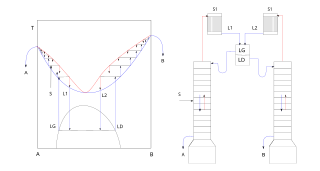
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, azeotropic distillation usually refers to the specific technique of adding another component to generate a new, lower-boiling azeotrope that is heterogeneous, such as the example below with the addition of benzene to water and ethanol.

Extractive distillation is defined as distillation in the presence of a miscible, high-boiling, relatively non-volatile component, the solvent, that forms no azeotrope with the other components in the mixture. The method is used for mixtures having a low value of relative volatility, nearing unity. Such mixtures cannot be separated by simple distillation, because the volatility of the two components in the mixture is nearly the same, causing them to evaporate at nearly the same temperature at a similar rate, making normal distillation impractical.
Reactive distillation is a process where the chemical reactor is also the still. Separation of the product from the reaction mixture does not need a separate distillation step which saves energy and materials. This technique can be useful for equilibrium-limited reactions such as esterification and ester hydrolysis reactions. Conversion can be increased beyond what is expected by the equilibrium due to the continuous removal of reaction products from the reactive zone. This approach can also reduce capital and investment costs.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
The Marcusson apparatus, Dean-Stark apparatus, Dean–Stark receiver, distilling trap, or Dean–Stark Head is a piece of laboratory glassware used in synthetic chemistry to collect water from a reactor. It is used in combination with a reflux condenser and a distillation flask for the separation of water from liquids. This may be a continuous removal of the water that is produced during a chemical reaction performed at reflux temperature, such as in esterification reactions. The original setup by Julius Marcusson was refined by the American chemists Ernest Woodward Dean (1888–1959) and David Dewey Stark (1893–1979) in 1920 for determination of the water content in petroleum.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
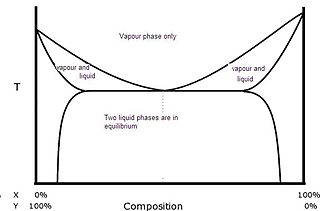
A heteroazeotrope is an azeotrope where the vapour phase coexists with two liquid phases. Sketch of a T-x/y equilibrium curve of a typical heteroazeotropic mixture
The McCabe–Thiele method is a technique that is commonly employed in the field of chemical engineering to model the separation of two substances by a distillation column. It uses the fact that the composition at each theoretical tray is completely determined by the mole fraction of one of the two components. This method is based on the assumptions that the distillation column is isobaric - i.e the pressure remains constant - and that the flow rates of liquid and vapor do not change throughout the column. The assumption of constant molar overflow requires that:
This page contains tables of azeotrope data for various binary and ternary mixtures of solvents. The data include the composition of a mixture by weight, the boiling point (b.p.) of a component, the boiling point of a mixture, and the specific gravity of the mixture. Boiling points are reported at a pressure of 760 mm Hg unless otherwise stated. Where the mixture separates into layers, values are shown for upper (U) and lower (L) layers.
Salt-effect distillation is a method of extractive distillation in which a salt is dissolved in the mixture of liquids to be distilled. The salt acts as a separating agent by raising the relative volatility of the mixture and by breaking any azeotropes that may otherwise form.
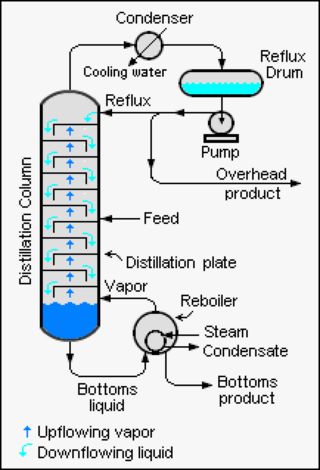
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

A residue curve describes the change in the composition of the liquid phase of a chemical mixture during continuous evaporation at the condition of vapor–liquid equilibrium. Multiple residue curves for a single system are called residue curves map.

Dmitri Petrovich Konovalov was a Russian-Soviet physical chemist who worked on gas-liquid phases of solutions in equilibrium and came up with several rules that were also independently worked on by J. Willard Gibbs and the rules are often called Gibbs-Konovalov rules. They provide the basis for distillation and separation of components that form azeotropes.