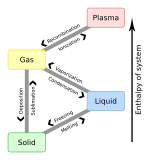
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.

In physics, a vapor or vapour is a substance in the gas phase at a temperature lower than its critical temperature, which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.

Dew is water in the form of droplets that appears on thin, exposed objects in the morning or evening due to condensation.

The dew point of a given body of air is the temperature to which it must be cooled to become saturated with water vapor. This temperature depends on the pressure and water content of the air. When the air is cooled below the dew point, its moisture capacity is reduced and airborne water vapor will condense to form liquid water known as dew. When this occurs through the air's contact with a colder surface, dew will form on that surface.

Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influenced by nearby bodies of water, topography, and wind conditions. In turn, fog affects many human activities, such as shipping, travel, and warfare.

Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds and fog.

A dehumidifier is an air conditioning device which reduces and maintains the level of humidity in the air. This is done usually for health or thermal comfort reasons, or to eliminate musty odor and to prevent the growth of mildew by extracting water from the air. It can be used for household, commercial, or industrial applications. Large dehumidifiers are used in commercial buildings such as indoor ice rinks and swimming pools, as well as manufacturing plants or storage warehouses. Typical air conditioning systems combine dehumidification with cooling, by operating cooling coils below the dewpoint and draining away the water that condenses.

An evaporative cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning systems, which use vapor-compression or absorption refrigeration cycles. Evaporative cooling exploits the fact that water will absorb a relatively large amount of heat in order to evaporate. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation). This can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of convenient heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.
An atmospheric water generator (AWG), is a device that extracts water from humid ambient air, producing potable water. Water vapor in the air can be extracted either by condensation - cooling the air below its dew point, exposing the air to desiccants, using membranes that only pass water vapor, collecting fog, or pressurizing the air. AWGs are useful where potable water is difficult to obtain, because water is always present in ambient air.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer, which provides the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.

In systems involving heat transfer, a condenser is a heat exchanger used to condense a gaseous substance into a liquid state through cooling. In so doing, the latent heat is released by the substance and transferred to the surrounding environment. Condensers are used for efficient heat rejection in many industrial systems. Condensers can be made according to numerous designs and come in many sizes ranging from rather small (hand-held) to very large. For example, a refrigerator uses a condenser to get rid of heat extracted from the interior of the unit to the outside air.

Pumpable icetechnology (PIT) uses thin liquids, with the cooling capacity of ice. Pumpable ice is typically a slurry of ice crystals or particles ranging from 5 micrometers to 1 cm in diameter and transported in brine, seawater, food liquid, or gas bubbles of air, ozone, or carbon dioxide.
In refrigeration, flash-gas is refrigerant in gas form produced spontaneously when the condensed liquid is subjected to boiling. The presence of flash-gas in the liquid lines reduces the efficiency of the refrigeration cycle. It can also lead several expansion systems to work improperly, and increase superheating at the evaporator. This is normally perceived as an unwanted condition caused by dissociation between the volume of the system, and the pressures and temperatures that allow the refrigerant to be liquid. Flash-gas must not be confused with lack of condensation, but special gear such as receivers, internal heat exchangers, insulation, and refrigeration cycle optimizers may improve condensation and avoid gas in the liquid lines.
In dropwise condensation the condensate liquid collects in the form of countless droplets of varying diameters on the condensing surface, instead of forming a continuous film, and does not wet the solid cooling surface. The droplets develop at points of surface imperfections, called nucleation sites, and grow in size as more vapour condenses on its exposed surface. When the size of droplets is large there comes a time the droplet breakaway from the surface and knock off other droplets and carries it downstream. The moving droplet devours the droplets of smaller size. Dropwise condensation is one of the most effective mechanism of heat transfer and extremely large heat transfer coefficients can be achieved with this mechanism. In dropwise condensation, there is no liquid film to resist heat transfer, and as a result heat transfer coefficients can be achieved more than 10 times larger than those associated with film condensation, although 3-5 times is more common. Heat transfer coefficients are large so designers can achieve a specified heat transfer rate with a smaller surface area and thus a smaller and less expensive condenser.
Compressed air dryers are special types of filter systems that are specifically designed to remove the water that is inherent in compressed air. The compression of air raises its temperature and concentrates atmospheric contaminants, primarily water vapor, as resulting in air with elevated temperature and 100% relative humidity. As the compressed air cools down, water vapor condenses into the tank(s), pipes, hoses and tools connected downstream from the compressor which may be damaging. Therefore water vapor is removed from compressed air to prevent condensation from occurring and to prevent moisture from interfering in sensitive industrial processes.
Heat engines, refrigeration cycles and heat pumps usually involve a fluid to and from which heat is transferred while undergoing a thermodynamic cycle. This fluid is called the working fluid. Refrigeration and heat pump technologies often refer to working fluids as refrigerants. Most thermodynamic cycles make use of the latent heat of the working fluid. In case of other cycles the working fluid remains in gaseous phase while undergoing all the processes of the cycle. When it comes to heat engines, working fluid generally undergoes a combustion process as well, for example in internal combustion engines or gas turbines. There are also technologies in heat pump and refrigeration, where working fluid does not change phase, such as reverse Brayton or Stirling cycle.
The low-temperature distillation (LTD) technology is the first implementation of the direct spray distillation (DSD) process. The first large-scale units are now in operation for desalination. The process was first developed by scientists at the University of Applied Sciences in Switzerland, focusing on low-temperature distillation in vacuum conditions, from 2000 to 2005.