Frost is a thin layer of ice on a solid surface, which forms from water vapor that deposits onto a freezing surface. Frost forms when the air contains more water vapor than it can normally hold at a specific temperature. The process is similar to the formation of dew, except it occurs below the freezing point of water typically without crossing through a liquid state.

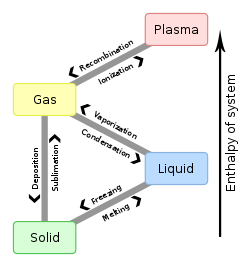
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.

A eutectic system or eutectic mixture is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the eutectic temperature. On a phase diagram, the eutectic temperature is seen as the eutectic point.

Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid or the liquid content of a substance, usually due to cooling.

The Mpemba effect is the name given to the observation that a liquid which is initially hot can freeze faster than the same liquid which begins cold, under otherwise similar conditions. There is disagreement about its theoretical basis and the parameters required to produce the effect.
In mathematics and its applications, particularly to phase transitions in matter, a Stefan problem is a particular kind of boundary value problem for a system of partial differential equations (PDE), in which the boundary between the phases can move with time. The classical Stefan problem aims to describe the evolution of the boundary between two phases of a material undergoing a phase change, for example the melting of a solid, such as ice to water. This is accomplished by solving heat equations in both regions, subject to given boundary and initial conditions. At the interface between the phases the temperature is set to the phase change temperature. To close the mathematical system a further equation, the Stefan condition, is required. This is an energy balance which defines the position of the moving interface. Note that this evolving boundary is an unknown (hyper-)surface; hence, Stefan problems are examples of free boundary problems.
In physics and chemistry, flash freezing is the process whereby objects are frozen in just a few hours by subjecting them to cryogenic temperatures, or through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.

Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation.

In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled below 0 °C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0 °C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay.
The Wegener–Bergeron–Findeisen process, is a process of ice crystal growth that occurs in mixed phase clouds in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.
Critical radius is the minimum particle size from which an aggregate is thermodynamically stable. In other words, it is the lowest radius formed by atoms or molecules clustering together before a new phase inclusion is viable and begins to grow. Formation of such stable nuclei is called nucleation.

A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regard to material properties.
Premelting refers to a quasi-liquid film that can occur on the surface of a solid even below melting point. The thickness of the film is temperature dependent. This effect is common for all crystalline materials. Premelting shows its effects in frost heave, and, taking grain boundary interfaces into account, maybe even in the movement of glaciers.
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin.

Insect winter ecology describes the overwinter survival strategies of insects, which are in many respects more similar to those of plants than to many other animals, such as mammals and birds. Unlike those animals, which can generate their own heat internally (endothermic), insects must rely on external sources to provide their heat (ectothermic). Thus, insects persisting in winter weather must tolerate freezing or rely on other mechanisms to avoid freezing. Loss of enzymatic function and eventual freezing due to low temperatures daily threatens the livelihood of these organisms during winter. Not surprisingly, insects have evolved a number of strategies to deal with the rigors of winter temperatures in places where they would otherwise not survive.

In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure.
The Gibbs–Thomson effect, in common physics usage, refers to variations in vapor pressure or chemical potential across a curved surface or interface. The existence of a positive interfacial energy will increase the energy required to form small particles with high curvature, and these particles will exhibit an increased vapor pressure. See Ostwald–Freundlich equation. More specifically, the Gibbs–Thomson effect refers to the observation that small crystals are in equilibrium with their liquid melt at a lower temperature than large crystals. In cases of confined geometry, such as liquids contained within porous media, this leads to a depression in the freezing point / melting point that is inversely proportional to the pore size, as given by the Gibbs–Thomson equation.
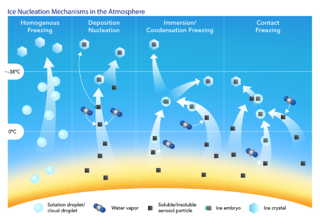
An ice nucleus, also known as an ice nucleating particle (INP), is a particle which acts as the nucleus for the formation of an ice crystal in the atmosphere.

Freeze-casting, also frequently referred to as ice-templating, or freeze alignment, is a technique that exploits the highly anisotropic solidification behavior of a solvent in a well-dispersed slurry to controllably template a directionally porous ceramic. By subjecting an aqueous slurry to a directional temperature gradient, ice crystals will nucleate on one side of the slurry and grow along the temperature gradient. The ice crystals will redistribute the suspended ceramic particles as they grow within the slurry, effectively templating the ceramic.
Classical nucleation theory (CNT) is the most common theoretical model used to quantitatively study the kinetics of nucleation.