
Glass is a non-crystalline solid that is often transparent, brittle and chemically inert. It has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics.

An amorphous metal is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity and can show metallic luster.
Phase-change memory is a type of non-volatile random-access memory. PRAMs exploit the unique behaviour of chalcogenide glass. In PCM, heat produced by the passage of an electric current through a heating element generally made of titanium nitride is used to either quickly heat and quench the glass, making it amorphous, or to hold it in its crystallization temperature range for some time, thereby switching it to a crystalline state. PCM also has the ability to achieve a number of distinct intermediary states, thereby having the ability to hold multiple bits in a single cell, but the difficulties in programming cells in this way has prevented these capabilities from being implemented in other technologies with the same capability.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.
GeSbTe (germanium-antimony-tellurium or GST) is a phase-change material from the group of chalcogenide glasses used in rewritable optical discs and phase-change memory applications. Its recrystallization time is 20 nanoseconds, allowing bitrates of up to 35 Mbit/s to be written and direct overwrite capability up to 106 cycles. It is suitable for land-groove recording formats. It is often used in rewritable DVDs. New phase-change memories are possible using n-doped GeSbTe semiconductor. The melting point of the alloy is about 600 °C (900 K) and the crystallization temperature is between 100 and 150 °C.
AgInSbTe, or silver-indium-antimony-tellurium, is a phase change material from the group of chalcogenide glasses, used in rewritable optical discs and phase-change memory applications. It is a quaternary compound of silver, indium, antimony, and tellurium.

Germanium telluride (GeTe) is a chemical compound of germanium and tellurium and is a component of chalcogenide glasses. It shows semimetallic conduction and ferroelectric behaviour.
The indium chalcogenides include all compounds of indium with the chalcogen elements, oxygen, sulfur, selenium and tellurium. (Polonium is excluded as little is known about its compounds with indium). The best-characterised compounds are the In(III) and In(II) chalcogenides e.g. the sulfides In2S3 and InS.
This group of compounds has attracted a lot of research attention because they include semiconductors, photovoltaics and phase-change materials. In many applications indium chalcogenides are used as the basis of ternary and quaternary compounds such as indium tin oxide, ITO and copper indium gallium selenide, CIGS.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

Thin-film solar cells are made by depositing one or more thin layers of photovoltaic material onto a substrate, such as glass, plastic or metal. Thin-film solar cells are typically a few nanometers (nm) to a few microns (µm) thick–much thinner than the wafers used in conventional crystalline silicon (c-Si) based solar cells, which can be up to 200 µm thick. Thin-film solar cells are commercially used in several technologies, including cadmium telluride (CdTe), copper indium gallium diselenide (CIGS), and amorphous thin-film silicon.

The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
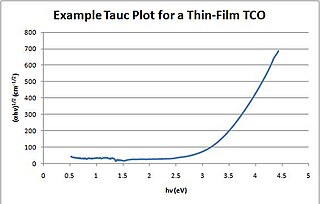
A Tauc plot is used to determine the optical bandgap, or Tauc bandgap, of either disordered or amorphous semiconductors.

Antimony telluride is an inorganic compound with the chemical formula Sb2Te3. As is true of other pnictogen chalcogenide layered materials, it is a grey crystalline solid with layered structure. Layers consist of two atomic sheets of antimony and three atomic sheets of tellurium and are held together by weak van der Waals forces. Sb2Te3 is a narrow-gap semiconductor with a band gap 0.21 eV; it is also a topological insulator, and thus exhibits thickness-dependent physical properties.

Wahid Shams-Kolahi is a scientist and an electrical engineer who is known for his research in photovoltaic-related technologies.
Gallium lanthanum sulfide glass is the name of a family of chalcogenide glasses, referred to as gallium lanthanum sulfide (Ga-La-S) glasses. They are mixtures of La2S3, La2O3, and Ga2S3, which form the basic glass with other glass modifiers added as needed. Gallium-lanthanum-sulfide glasses have a wide range of vitreous formation centered around a 70% Ga2S3 : 30% La2S3 mixture, and readily accept other modifier materials into their structure. This means that Ga-La-S composition can be adjusted to give a wide variety of optical and physical properties.
Chalcogenide chemical vapour deposition is a proposed technology for depositing thin films of chalcogenides, i.e. materials derived from sulfides, selenides, and tellurides. Conventional CVD can be used to deposit films of most metals, many non-metallic elements as well as a large number of compounds including carbides, nitrides, oxides. CVD can be used to synthesize chalcogenide glasses.
The electron-refractive effect, also known as electron induced permittivity modification (EIPM), is an electro-optic effect observed in some crystals and amorphous materials, such as chalcogenide glasses and oxides, where the permittivity reduces or increases when the material is illuminated by high energy electrons, typically from a transmission electron microscope or scanning electron microscope. The effect is non-linear and reversible.
Rigidity theory, or topological constraint theory, is a tool for predicting properties of complex networks based on their composition. It was introduced by James Charles Phillips in 1979 and 1981, and refined by Michael Thorpe in 1983. Inspired by the study of the stability of mechanical trusses as pioneered by James Clerk Maxwell, and by the seminal work on glass structure done by William Houlder Zachariasen, this theory reduces complex molecular networks to nodes constrained by rods, thus filtering out microscopic details that ultimately don't affect macroscopic properties. An equivalent theory was developed by P.K. Gupta A.R. Cooper in 1990, where rather than nodes representing atoms, they represented unit polytopes. An example of this would be the SiO tetrahedra in pure glassy silica. This style of analysis has applications in biology and chemistry, such as understanding adaptability in protein-protein interaction networks. Rigidity theory applied to the molecular networks arising from phenotypical expression of certain diseases may provide insights regarding their structure and function.
Arsenic(III) telluride is an inorganic compound with the chemical formula As2Te3. It exists in two forms, the monoclinic α phase which transforms under high pressure to a rhombohedral β phase. The compound is a semiconductor, with most current carried by holes. Arsenic telluride has been examined for its use in nonlinear optics.










