Jaundice, also known as icterus, is a yellowish or greenish pigmentation of the skin and sclera due to high bilirubin levels. Jaundice in adults is typically a sign indicating the presence of underlying diseases involving abnormal heme metabolism, liver dysfunction, or biliary-tract obstruction. The prevalence of jaundice in adults is rare, while jaundice in babies is common, with an estimated 80% affected during their first week of life. The most commonly associated symptoms of jaundice are itchiness, pale feces, and dark urine.

Bilirubin (BR) is a red-orange compound that occurs in the normal catabolic pathway that breaks down heme in vertebrates. This catabolism is a necessary process in the body's clearance of waste products that arise from the destruction of aged or abnormal red blood cells. In the first step of bilirubin synthesis, the heme molecule is stripped from the hemoglobin molecule. Heme then passes through various processes of porphyrin catabolism, which varies according to the region of the body in which the breakdown occurs. For example, the molecules excreted in the urine differ from those in the feces. The production of biliverdin from heme is the first major step in the catabolic pathway, after which the enzyme biliverdin reductase performs the second step, producing bilirubin from biliverdin.

Bile, or gall, is a yellow-green fluid produced by the liver of most vertebrates that aids the digestion of lipids in the small intestine. In humans, bile is primarily composed of water, produced continuously by the liver, and stored and concentrated in the gallbladder. After a human eats, this stored bile is discharged into the first section of their small intestine.

Heme, or haem, is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver.

Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism. It is the chemical responsible for the brown color of human feces and was originally isolated from feces in 1932. Stercobilin can be used as a marker for biochemical identification of fecal pollution levels in rivers.
Phycobilins are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis.

Hereditary spherocytosis (HS) is a congenital hemolytic disorder wherein a genetic mutation coding for a structural membrane protein phenotype causes the red blood cells to be sphere-shaped (spherocytosis), rather than the normal biconcave disk shape. This abnormal shape interferes with the cells' ability to flex during blood circulation, and also makes them more prone to rupture under osmotic stress, mechanical stress, or both. Cells with the dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes and results in hemolytic anemia.

Phytochromes are a class of photoreceptor proteins found in plants, bacteria and fungi. They respond to light in the red and far-red regions of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light. Recent advances have suggested that phytochromes also act as temperature sensors, as warmer temperatures enhance their de-activation. All of these factors contribute to the plant's ability to germinate.

Heme oxygenase, or haem oxygenase, is an enzyme that catalyzes the degradation of heme to produce biliverdin, ferrous ion, and carbon monoxide.

Biliverdin reductase (BVR) is an enzyme found in all tissues under normal conditions, but especially in reticulo-macrophages of the liver and spleen. BVR facilitates the conversion of biliverdin to bilirubin via the reduction of a double bond between the second and third pyrrole ring into a single bond.

Urobilin or urochrome is the chemical primarily responsible for the yellow color of urine. It is a linear tetrapyrrole compound that, along with the related colorless compound urobilinogen, are degradation products of the cyclic tetrapyrrole heme.
Neonatal cholestasis refers to elevated levels of conjugated bilirubin identified in newborn infants within the first few months of life. Conjugated hyperbilirubinemia is clinically defined as >20% of total serum bilirubin or conjugated bilirubin concentration greater than 1.0 mg/dL regardless of total serum bilirubin concentration. The differential diagnosis for neonatal cholestasis can vary extensively. However, the underlying disease pathology is caused by improper transport and/or defects in excretion of bile from hepatocytes leading to an accumulation of conjugated bilirubin in the body. Generally, symptoms associated with neonatal cholestasis can vary based on the underlying cause of the disease. However, most infants affected will present with jaundice, scleral icterus, failure to thrive, acholic or pale stools, and dark urine.

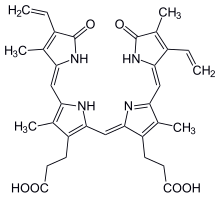
Bilins, bilanes or bile pigments are biological pigments formed in many organisms as a metabolic product of certain porphyrins. Bilin was named as a bile pigment of mammals, but can also be found in lower vertebrates, invertebrates, as well as red algae, green plants and cyanobacteria. Bilins can range in color from red, orange, yellow or brown to blue or green.
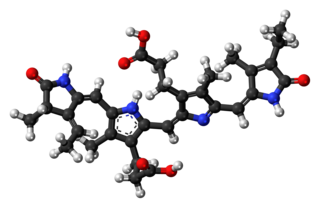
Phycocyanobilin is a blue phycobilin, i.e., a tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes, and some cryptomonads. Phycocyanobilin is present only in the phycobiliproteins allophycocyanin and phycocyanin, of which it is the terminal acceptor of energy. It is covalently linked to these phycobiliproteins by a thioether bond.

Roger Yonchien Tsien was an American biochemist. He was a professor of chemistry and biochemistry at the University of California, San Diego and was awarded the Nobel Prize in Chemistry in 2008 for his discovery and development of the green fluorescent protein, in collaboration with organic chemist Osamu Shimomura and neurobiologist Martin Chalfie. Tsien was also a pioneer of calcium imaging.
A chromoprotein is a conjugated protein that contains a pigmented prosthetic group. A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other examples of chromoproteins include other hemochromes, cytochromes, phytochromes and flavoproteins.

Small ultra red fluorescent protein (smURFP) is a class of far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein, α-allophycocyanin. Native α-allophycocyanin requires an exogenous protein, known as a lyase, to attach the chromophore, phycocyanobilin. Phycocyanobilin is not present in mammalian cells. smURFP was evolved to covalently attach phycocyanobilin without a lyase and fluoresce, covalently attach biliverdin and fluoresce, blue-shift fluorescence to match the organic fluorophore, Cy5, and not inhibit E. coli growth. smURFP was found after 12 rounds of random mutagenesis and manually screening 10,000,000 bacterial colonies.

Biliproteins are pigment protein compounds that are located in photosynthesising organisms such as algae, and sometimes also in certain insects. They refer to any protein that contains a bilin chromophore. In plants and algae, the main function of biliproteins is to make the process of light accumulation required for photosynthesis more efficient; while in insects they play a role in growth and development. Some of their properties: including light-receptivity, light-harvesting and fluorescence have made them suitable for applications in bioimaging and as indicators; while other properties such as anti-oxidation, anti-aging and anti-inflammation in phycobiliproteins have given them potential for use in medicine, cosmetics and food technology. While research on biliproteins dates back as far as 1950, it was hindered due to issues regarding biliprotein structure, lack of methods available for isolating individual biliprotein components, as well as limited information on lyase reactions . Research on biliproteins has also been primarily focused on phycobiliproteins; but advances in technology and methodology, along with the discovery of different types of lyases, has renewed interest in biliprotein research, allowing new opportunities for investigating biliprotein processes such as assembly/disassembly and protein folding.
Hemolytic jaundice, also known as prehepatic jaundice, is a type of jaundice arising from hemolysis or excessive destruction of red blood cells, when the byproduct bilirubin is not excreted by the hepatic cells quickly enough. Unless the patient is concurrently affected by hepatic dysfunctions or is experiencing hepatocellular damage, the liver does not contribute to this type of jaundice.
Alexander Glazer was a professor of the Graduate School in the Department of Molecular and Cell Biology at the University of California, Berkeley. He had a passion for protein chemistry and structure function relationships. He also had a longstanding interest in light-harvesting complexes in cyanobacteria and red algae called phycobilisomes. He had also spent more than 10 years working on the human genome project where he has investigated methods for DNA detection and sequencing which most notably includes the development of fluorescent reagents involved in cell labeling. Most recently, he had focused his studies on issues in environmental sciences. He died on July 18, 2021, in Orinda, California