
An excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons. In this case, formation of molecules is possible only if such atom is in an electronic excited state. Heteronuclear molecules and molecules that have more than two species are also called exciplex molecules. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is very short, on the order of nanoseconds.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).
Hyperpolarization is the nuclear spin polarization of a material in a magnetic field far beyond thermal equilibrium conditions determined by the Boltzmann distribution. It can be applied to gases such as 129Xe and 3He, and small molecules where the polarization levels can be enhanced by a factor of 104-105 above thermal equilibrium levels. Hyperpolarized noble gases are typically used in magnetic resonance imaging (MRI) of the lungs. Hyperpolarized small molecules are typically used for in vivo metabolic imaging. For example, a hyperpolarized metabolite can be injected into animals or patients and the metabolic conversion can be tracked in real-time. Other applications include determining the function of the neutron spin-structures by scattering polarized electrons from a very polarized target (3He), surface interaction studies, and neutron polarizing experiments.
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. This spectroscopy is based on the measurement of absorption of electromagnetic radiations in the radio frequency region from roughly 4 to 900 MHz. Absorption of radio waves in the presence of magnetic field is accompanied by a special type of nuclear transition, and for this reason, such type of spectroscopy is known as Nuclear Magnetic Resonance Spectroscopy. The sample is placed in a magnetic field and the NMR signal is produced by excitation of the nuclei sample with radio waves into nuclear magnetic resonance, which is detected with sensitive radio receivers. The intramolecular magnetic field around an atom in a molecule changes the resonance frequency, thus giving access to details of the electronic structure of a molecule and its individual functional groups. As the fields are unique or highly characteristic to individual compounds, in modern organic chemistry practice, NMR spectroscopy is the definitive method to identify monomolecular organic compounds.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization and the polarization of the wave must match the orientation of the magnetization.
In MRI and NMR spectroscopy, an observable nuclear spin polarization (magnetization) is created by a homogeneous magnetic field. This field makes the magnetic dipole moments of the sample precess at the resonance (Larmor) frequency of the nuclei. At thermal equilibrium, nuclear spins precess randomly about the direction of the applied field. They become abruptly phase coherent when they are hit by radiofrequency (RF) pulses at the resonant frequency, created orthogonal to the field. The RF pulses cause the population of spin-states to be perturbed from their thermal equilibrium value. The generated transverse magnetization can then induce a signal in an RF coil that can be detected and amplified by an RF receiver. The return of the longitudinal component of the magnetization to its equilibrium value is termed spin-latticerelaxation while the loss of phase-coherence of the spins is termed spin-spin relaxation, which is manifest as an observed free induction decay (FID).
Spin chemistry is a sub-field of chemistry positioned at the intersection of chemical kinetics, photochemistry, magnetic resonance and free radical chemistry, that deals with magnetic and spin effects in chemical reactions. Spin chemistry concerns phenomena such as chemically induced dynamic nuclear polarization (CIDNP), chemically induced electron polarization (CIDEP), magnetic isotope effects in chemical reactions, and it is hypothesized to be key in the underlying mechanism for avian magnetoreception and consciousness.
Peter John Hore is a British chemist and academic. He is a Professor of Chemistry at the University of Oxford and fellow of Corpus Christi College, Oxford. He is the author of two Oxford Chemistry Primers on Nuclear Magnetic Resonance (NMR) and research articles primarily in the area of NMR, electron paramagnetic resonance (EPR), spin chemistry and magnetoreception during bird migration.

Spin trapping is an analytical technique employed in chemistry and biology for the detection and identification of short-lived free radicals through the use of electron paramagnetic resonance (EPR) spectroscopy. EPR spectroscopy detects paramagnetic species such as the unpaired electrons of free radicals. However, when the half-life of radicals is too short to detect with EPR, compounds known as spin traps are used to react covalently with the radical products and form more stable adduct that will also have paramagnetic resonance spectra detectable by EPR spectroscopy. The use of radical-addition reactions to detect short-lived radicals was developed by several independent groups by 1968.

Zero- to ultralow-field (ZULF) NMR is the acquisition of nuclear magnetic resonance (NMR) spectra of chemicals with magnetically active nuclei in an environment carefully screened from magnetic fields. ZULF NMR experiments typically involve the use of passive or active shielding to attenuate Earth’s magnetic field. This is in contrast to the majority of NMR experiments which are performed in high magnetic fields provided by superconducting magnets. In ZULF experiments the dominant interactions are nuclear spin-spin couplings, and the coupling between spins and the external magnetic field is a perturbation to this. There are a number of advantages to operating in this regime: magnetic-susceptibility-induced line broadening is attenuated which reduces inhomogeneous broadening of the spectral lines for samples in heterogeneous environments. Another advantage is that the low frequency signals readily pass through conductive materials such as metals due to the increased skin depth; this is not the case for high-field NMR for which the sample containers are usually made of glass, quartz or ceramic.
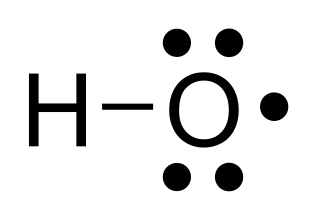
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.

Paramagnetic nuclear magnetic resonance spectroscopy refers to nuclear magnetic resonance (NMR) spectroscopy of paramagnetic compounds. Although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals. Paramagnetism diminishes the resolution of an NMR spectrum to the extent that coupling is rarely resolved. Nonetheless spectra of paramagnetic compounds provide insight into the bonding and structure of the sample. For example, the broadening of signals is compensated in part by the wide chemical shift range (often 200 ppm in 1H NMR). Since paramagnetism leads to shorter relaxation times (T1), the rate of spectral acquisition can be high.

Spectroelectrochemistry (SEC) is a set of multi-response analytical techniques in which complementary chemical information is obtained in a single experiment. Spectroelectrochemistry provides a whole vision of the phenomena that take place in the electrode process. The first spectroelectrochemical experiment was carried out by Theodore Kuwana, PhD, in 1964.
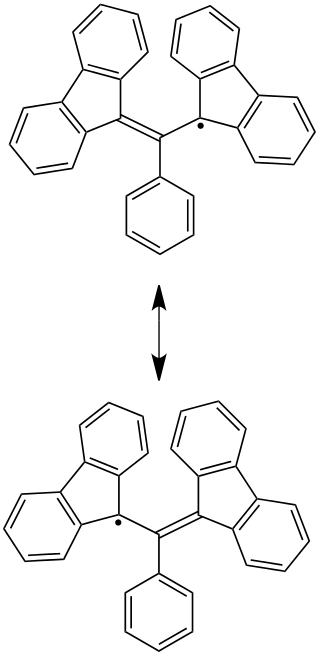
The Koelsch radical is a chemical compound that is an unusually stable carbon-centered radical, due to its resonance structures.
Anne Lesage is a French engineer who is a group leader at the French National Centre for Scientific Research. She is based at the High Field NMR Centre of the Lyon Institute of Analytical Sciences, where she develops novel nuclear magnetic resonance approaches to characterise solid-state materials.











