
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice. Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth. Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans.

Clathrate hydrates, or gas hydrates, clathrates, or hydrates, are crystalline water-based solids physically resembling ice, in which small non-polar molecules or polar molecules with large hydrophobic moieties are trapped inside "cages" of hydrogen bonded, frozen water molecules. In other words, clathrate hydrates are clathrate compounds in which the host molecule is water and the guest molecule is typically a gas or liquid. Without the support of the trapped molecules, the lattice structure of hydrate clathrates would collapse into conventional ice crystal structure or liquid water. Most low molecular weight gases, including O2, H2, N2, CO2, CH4, H2S, Ar, Kr, and Xe, as well as some higher hydrocarbons and freons, will form hydrates at suitable temperatures and pressures. Clathrate hydrates are not officially chemical compounds, as the enclathrated guest molecules are never bonded to the lattice. The formation and decomposition of clathrate hydrates are first order phase transitions, not chemical reactions. Their detailed formation and decomposition mechanisms on a molecular level are still not well understood. Clathrate hydrates were first documented in 1810 by Sir Humphry Davy who found that water was a primary component of what was earlier thought to be solidified chlorine.

Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue (other than incidental frost from moisture in the atmosphere). It is useful for preserving frozen foods (such as ice cream) where mechanical cooling is unavailable.

The atmosphere of Mars is the layer of gases surrounding Mars. It is primarily composed of carbon dioxide (95%), molecular nitrogen (2.8%), and argon (2%). It also contains trace levels of water vapor, oxygen, carbon monoxide, hydrogen, and noble gases. The atmosphere of Mars is much thinner than Earth's. The average surface pressure is only about 610 pascals (0.088 psi) which is less than 1% of the Earth's value. The currently thin Martian atmosphere prohibits the existence of liquid water on the surface of Mars, but many studies suggest that the Martian atmosphere was much thicker in the past. The higher density during spring and fall is reduced by 25% during the winter when carbon dioxide partly freezes at the pole caps. The highest atmospheric density on Mars is equal to the density found 35 km (22 mi) above the Earth's surface and is ≈0.020 kg/m3. The atmosphere of Mars has been losing mass to space since the planet's core slowed down, and the leakage of gases still continues today. The atmosphere of Mars is colder than Earth's. Owing to the larger distance from the Sun, Mars receives less solar energy and has a lower effective temperature, which is about 210 K. The average surface emission temperature of Mars is just 215 K, which is comparable to inland Antarctica. Although Mars' atmosphere consists primarily of carbon dioxide, the greenhouse effect in the Martian atmosphere is much weaker than Earth's: 5 °C (9.0 °F) on Mars, versus 33 °C (59 °F) on Earth. This is because the total atmosphere is so thin that the partial pressure of carbon dioxide is very weak, leading to less warming. The daily range of temperature in the lower atmosphere is huge due to the low thermal inertia; it can range from −75 °C (−103 °F) to near 0 °C (32 °F) near the surface in some regions. The temperature of the upper part of the Martian atmosphere is also significantly lower than Earth's because of the absence of stratospheric ozone and the radiative cooling effect of carbon dioxide at higher altitudes.

The terraforming of Mars or the terraformation of Mars is a hypothetical procedure that would consist of a planetary engineering project or concurrent projects, with the goal to transform Mars from a planet hostile to terrestrial life to one that can sustainably host humans and other lifeforms free of protection or mediation. The process would involve the modification of the planet's extant climate, atmosphere, and surface through a variety of resource-intensive initiatives, and the installation of a novel ecological system or systems.

The Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) was a visible-infrared spectrometer aboard the Mars Reconnaissance Orbiter searching for mineralogic indications of past and present water on Mars. The CRISM instrument team comprised scientists from over ten universities and was led by principal investigator Scott Murchie. CRISM was designed, built, and tested by the Johns Hopkins University Applied Physics Laboratory.

Planum Australe is the southern polar plain on Mars. It extends southward of roughly 75°S and is centered at 83.9°S 160.0°E. The geology of this region was to be explored by the failed NASA mission Mars Polar Lander, which lost contact on entry into the Martian atmosphere.

The climate of Mars has been a topic of scientific curiosity for centuries, in part because it is the only terrestrial planet whose surface can be easily directly observed in detail from the Earth with help from a telescope.
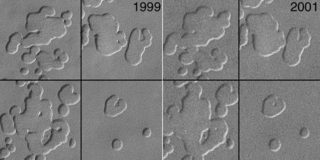
Swiss cheese features (SCFs) are curious pits in the south polar ice cap of Mars named from their similarity to the holes in Swiss cheese. They were first seen in 2000 using Mars Orbiter Camera imagery.

Martian geysers are putative sites of small gas and dust eruptions that occur in the south polar region of Mars during the spring thaw. "Dark dune spots" and "spiders" – or araneiforms – are the two most visible types of features ascribed to these eruptions.

The Mars ocean theory states that nearly a third of the surface of Mars was covered by an ocean of liquid water early in the planet's geologic history. This primordial ocean, dubbed Paleo-Ocean or Oceanus Borealis, would have filled the basin Vastitas Borealis in the northern hemisphere, a region which lies 4–5 km below the mean planetary elevation, at a time period of approximately 4.1–3.8 billion years ago. Evidence for this ocean includes geographic features resembling ancient shorelines, and the chemical properties of the Martian soil and atmosphere. Early Mars would have required a denser atmosphere and warmer climate to allow liquid water to remain at the surface.

The Mare Australe quadrangle is one of a series of 30 quadrangle maps of Mars used by the United States Geological Survey (USGS) Astrogeology Research Program. The Mare Australe quadrangle is also referred to as MC-30. The quadrangle covers all the area of Mars south of 65°, including the South polar ice cap, and its surrounding area. The quadrangle's name derives from an older name for a feature that is now called Planum Australe, a large plain surrounding the polar cap. The Mars polar lander crash landed in this region.

Almost all water on Mars today exists as ice, though it also exists in small quantities as vapor in the atmosphere. What was thought to be low-volume liquid brines in shallow Martian soil, also called recurrent slope lineae, may be grains of flowing sand and dust slipping downhill to make dark streaks. While most water ice is buried, it is exposed at the surface across several locations on Mars. In the mid-latitudes, it is exposed by impact craters, steep scarps and gullies. Additionally, water ice is also visible at the surface at the north polar ice cap. Abundant water ice is also present beneath the permanent carbon dioxide ice cap at the Martian south pole. More than 5 million km3 of ice have been detected at or near the surface of Mars, enough to cover the whole planet to a depth of 35 meters (115 ft). Even more ice might be locked away in the deep subsurface.

The planet Mars has two permanent polar ice caps. During a pole's winter, it lies in continuous darkness, chilling the surface and causing the deposition of 25–30% of the atmosphere into slabs of CO2 ice (dry ice). When the poles are again exposed to sunlight, the frozen CO2 sublimes. These seasonal actions transport large amounts of dust and water vapor, giving rise to Earth-like frost and large cirrus clouds.

Martian gullies are small, incised networks of narrow channels and their associated downslope sediment deposits, found on the planet of Mars. They are named for their resemblance to terrestrial gullies. First discovered on images from Mars Global Surveyor, they occur on steep slopes, especially on the walls of craters. Usually, each gully has a dendritic alcove at its head, a fan-shaped apron at its base, and a single thread of incised channel linking the two, giving the whole gully an hourglass shape. They are estimated to be relatively young because they have few, if any craters. A subclass of gullies is also found cut into the faces of sand dunes, that are themselves considered to be quite young. Linear dune gullies are now considered recurrent seasonal features.
Chaos terrain on Mars is distinctive; nothing on Earth compares to it. Chaos terrain generally consists of irregular groups of large blocks, some tens of kilometers across and a hundred or more meters high. The tilted and flat topped blocks form depressions hundreds of metres deep. A chaotic region can be recognized by a rat's nest of mesas, buttes, and hills, chopped through with valleys which in places look almost patterned. Some parts of this chaotic area have not collapsed completely—they are still formed into large mesas, so they may still contain water ice. Chaos regions formed long ago. By counting craters and by studying the valleys' relations with other geological features, scientists have concluded the channels formed 2.0 to 3.8 billion years ago.

A planetary surface is where the solid or liquid material of certain types of astronomical objects contacts the atmosphere or outer space. Planetary surfaces are found on solid objects of planetary mass, including terrestrial planets, dwarf planets, natural satellites, planetesimals and many other small Solar System bodies (SSSBs). The study of planetary surfaces is a field of planetary geology known as surface geology, but also a focus on a number of fields including planetary cartography, topography, geomorphology, atmospheric sciences, and astronomy. Land is the term given to non-liquid planetary surfaces. The term landing is used to describe the collision of an object with a planetary surface and is usually at a velocity in which the object can remain intact and remain attached.
Nitrogen clathrate or nitrogen hydrate is a clathrate consisting of ice with regular crystalline cavities that contain nitrogen molecules. Nitrogen clathrate is a variety of air hydrates. It occurs naturally in ice caps on Earth, and is believed to be important in the outer Solar System on moons such as Titan and Triton which have a cold nitrogen atmosphere.
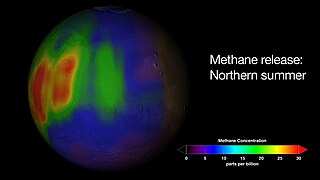
The reported presence of methane in the atmosphere of Mars is of interest to many geologists and astrobiologists, as methane may indicate the presence of microbial life on Mars, or a geochemical process such as volcanism or hydrothermal activity.
Mars's atmosphere is predominantly composed of CO2 (around 95%) with seasonal air pressure change that facilitates the vaporization and condensation of carbon dioxide. The CO2 cycle on the planet Mars has facilitated the formation of CO2 ice clouds at various locations and seasons on the red planet. Due to low temperatures, especially at Mars's polar caps, carbon dioxide gas can freeze in Mars’s atmosphere to form ice crystallized clouds. Several missions, such as the Viking, Mars Global Surveyor, and Mars Express, have led to interesting observations and measurements regarding CO2 ice clouds. MOLA data in addition to TES spectra have documented ice clouds forming during the winter season of Mars’s northern and southern polar caps. In addition, the Curiosity rover has imaged clouds well above 60 kilometers in the sky at the planet’s equator during the coldest time of Mars’s orbital year (when Mars is furthest away from the Sun due to its elliptical orbit), indicating the possibility of CO2 ice clouds around the planet’s equator. Although further data collection is needed to confirm the formation of CO2 ice clouds on Mars, especially at the planet’s equator, previous measurements have developed a strong argument for frozen carbon dioxide clouds on Mars.

















