
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.
Fungicides are pesticides used to kill parasitic fungi or their spores. They are most commonly chemical compounds, but may include biocontrols and fungistatics. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, despite their large differences in method of plant invasion. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward.

Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (malon) meaning 'apple'.

Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential.
Pyrazole is an organic compound of azole group with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with pKb 11.5 (pKa of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol.
The cereal grain wheat is subject to numerous wheat diseases, including bacterial, viral and fungal diseases, as well as parasitic infestations.
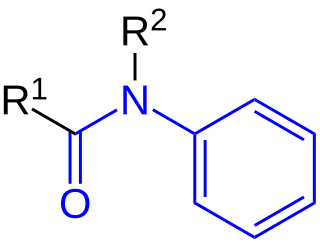
In organic chemistry, anilides are a class of organic compounds with the general structure R−C(=O)−N(−R’)−C6H5. They are amide derivatives of aniline.

Acibenzolar-S-methyl is the ISO common name for an organic compound that is used as a fungicide. Unusually, it is not directly toxic to fungi but works by inducing systemic acquired resistance, the natural defence system of plants.

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.

Saflufenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf weeds in crops including soybeans and corn.
Early twenty-first century pesticide research has focused on developing molecules that combine low use rates and that are more selective, safer, resistance-breaking and cost-effective. Obstacles include increasing pesticide resistance and an increasingly stringent regulatory environment.

Fluxapyroxad is a broad-spectrum pyrazole-carboxamide fungicide used on a large variety of commercial crops. It stunts fungus growth by inhibiting the succinate dehydrogenase (SQR) enzyme. Application of fluxapyroxad helps prevent many wilts and other fungal infections from taking hold. As with other systemic pesticides that have a long chemical half-life, there are concerns about keeping fluxapyroxad out of the groundwater, especially when combined with pyraclostrobin. There is also concern that some fungi may develop resistance to fluxapyroxad.
Fluopyram is a fungicide and nematicide used in agriculture. It is used to control fungal diseases such as gray mold (Botrytis), powdery mildew, apple scab, Alternaria, Sclerotinia, and Monilinia. It is an inhibitor of succinate dehydrogenase.

Tebufenpyrad is an insecticide and acaricide widely used in greenhouses. It is a white solid chemical with a slight aromatic smell. It is soluble in water and also in organic solvents.

Oxycarboxin is an organic chemical used in agriculture to protect crops from fungal diseases. It was first marketed by Uniroyal in 1969 using their brand name Plantvax. The compound is an anilide which combines a heterocyclic acid with aniline to give an inhibitor of succinate dehydrogenase (SDHI).
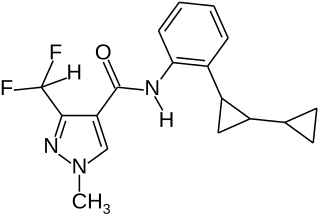
Sedaxane is a broad spectrum fungicide used as a seed treatment in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2011 using their brand name Vibrance. The compound is an amide which combines a pyrazole acid with a substituted aniline to give an inhibitor of succinate dehydrogenase.

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO) which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

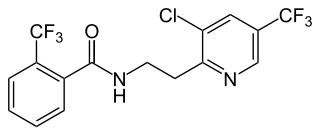
Pydiflumetofen is a broad spectrum fungicide used in agriculture to protect crops from fungal diseases. It was first marketed by Syngenta in 2016 using their brand name Miravis. The compound is an amide which combines a pyrazole acid with a substituted phenethylamine to give an inhibitor of succinate dehydrogenase.

3-(Difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid is a chemical compound which is used commercially as an intermediate to seven fungicides which act by inhibition of succinate dehydrogenase (SDHI). It consists of a pyrazole ring with difluoromethyl, methyl and carboxylic acid groups attached in specific positions.



















