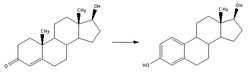
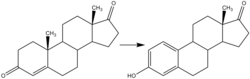
Estrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.

The menstrual cycle is a series of natural changes in hormone production and the structures of the uterus and ovaries of the female reproductive system that makes pregnancy possible. The ovarian cycle controls the production and release of eggs and the cyclic release of estrogen and progesterone. The uterine cycle governs the preparation and maintenance of the lining of the uterus (womb) to receive an embryo. These cycles are concurrent and coordinated, normally last between 21 and 35 days, with a median length of 28 days, and continue for about 30–45 years.

Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain.

Follicle-stimulating hormone (FSH) is a gonadotropin, a glycoprotein polypeptide hormone. FSH is synthesized and secreted by the gonadotropic cells of the anterior pituitary gland and regulates the development, growth, pubertal maturation, and reproductive processes of the body. FSH and luteinizing hormone (LH) work together in the reproductive system.

Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol.

Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens.
Bioidentical hormone replacement therapy (BHRT), also known as bioidentical hormone therapy (BHT) or natural hormone therapy, is the use of hormones that are identical on a molecular level with endogenous hormones in hormone replacement therapy. It may also be combined with blood and saliva testing of hormone levels, and the use of pharmacy compounding to obtain hormones in an effort to reach a targeted level of hormones in the body. A number of claims by some proponents of BHT have not been confirmed through scientific testing. Specific hormones used in BHT include estrone, estradiol, progesterone, testosterone, dehydroepiandrosterone (DHEA), and estriol.
Dydrogesterone, sold under the brand name Dydroboon & Duphaston among others, is a progestin medication which is used for a variety of indications, including threatened or recurrent miscarriage during pregnancy, dysfunctional bleeding, infertility due to luteal insufficiency, dysmenorrhea, endometriosis, secondary amenorrhea, irregular cycles, premenstrual syndrome, and as a component of menopausal hormone therapy. It is taken by mouth.

Estriol succinate, sold under the brand name Synapause among others, is an estrogen medication which is used in the treatment of menopausal symptoms. It is taken by mouth, in through the vagina, and by injection.

Nomegestrol acetate (NOMAC), sold under the brand names Lutenyl and Zoely among others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders. It is available both alone and in combination with an estrogen. NOMAC is taken by mouth. A birth control implant for placement under the skin was also developed but ultimately was not marketed.
Sexual motivation is influenced by hormones such as testosterone, estrogen, progesterone, oxytocin, and vasopressin. In most mammalian species, sex hormones control the ability and motivation to engage in sexual behaviours.
An estrogen-dependent condition can be that relating to the differentiation in the steroid sex hormone that is associated with the female reproductive system and sex characteristics. These conditions can fall under the umbrella of hypoestrogenism, hyperestrogenim, or any sensitivity to the presence of estrogen in the body.

Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of the sodium salts of estrogen conjugates found in horses, such as estrone sulfate and equilin sulfate. CEEs are available in the form of both natural preparations manufactured from the urine of pregnant mares and fully synthetic replications of the natural preparations. They are formulated both alone and in combination with progestins such as medroxyprogesterone acetate. CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.

Estradiol sulfate (E2S), or 17β-estradiol 3-sulfate, is a natural, endogenous steroid and an estrogen ester. E2S itself is biologically inactive, but it can be converted by steroid sulfatase into estradiol, which is a potent estrogen. Simultaneously, estrogen sulfotransferases convert estradiol to E2S, resulting in an equilibrium between the two steroids in various tissues. Estrone and E2S are the two immediate metabolic sources of estradiol. E2S can also be metabolized into estrone sulfate (E1S), which in turn can be converted into estrone and estradiol. Circulating concentrations of E2S are much lower than those of E1S. High concentrations of E2S are present in breast tissue, and E2S has been implicated in the biology of breast cancer via serving as an active reservoir of estradiol.

Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in feminizing hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.

Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle.

Estrone (E1), sold under the brand names Estragyn, Kestrin, and Theelin among many others, is an estrogen medication and naturally occurring steroid hormone which has been used in menopausal hormone therapy and for other indications. It has been provided as an aqueous suspension or oil solution given by injection into muscle and as a vaginal cream applied inside of the vagina. It can also be taken by mouth as estradiol/estrone/estriol and in the form of prodrugs like estropipate and conjugated estrogens.

Estetrol (E4) is an estrogen medication and naturally occurring steroid hormone which is used in combination with a progestin in combined birth control pills and is under development for various other indications. These investigational uses include menopausal hormone therapy to treat symptoms such as vaginal atrophy, hot flashes, and bone loss and the treatment of breast cancer and prostate cancer. It is taken by mouth.
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
The pharmacology of progesterone, a progestogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.