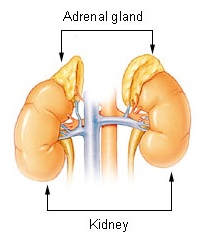
The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.
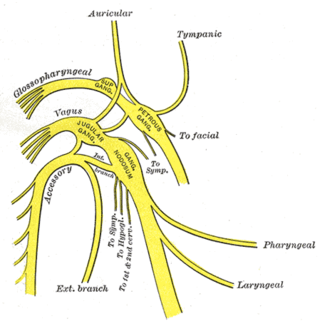
The vagus nerve, also known as the tenth cranial nerve, cranial nerve X, or simply CN X, is a cranial nerve that carries sensory fibers that create a pathway that interfaces with the parasympathetic control of the heart, lungs, and digestive tract. It comprises two nerves—the left and right vagus nerves—but they are typically referred to collectively as a single subsystem.

A catecholamine is a monoamine neurotransmitter, an organic compound that has a catechol and a side-chain amine.

The adrenal medulla is the inner part of the adrenal gland. It is located at the center of the gland, being surrounded by the adrenal cortex. It is the innermost part of the adrenal gland, consisting of chromaffin cells that secrete catecholamines, including epinephrine (adrenaline), norepinephrine (noradrenaline), and a small amount of dopamine, in response to stimulation by sympathetic preganglionic neurons.

Chromaffin cells, also called pheochromocytes, are neuroendocrine cells found mostly in the medulla of the adrenal glands in mammals. These cells serve a variety of functions such as serving as a response to stress, monitoring carbon dioxide and oxygen concentrations in the body, maintenance of respiration and the regulation of blood pressure. They are in close proximity to pre-synaptic sympathetic ganglia of the sympathetic nervous system, with which they communicate, and structurally they are similar to post-synaptic sympathetic neurons. In order to activate chromaffin cells, the splanchnic nerve of the sympathetic nervous system releases acetylcholine, which then binds to nicotinic acetylcholine receptors on the adrenal medulla. This causes the release of catecholamines. The chromaffin cells release catecholamines: ~80% of adrenaline (epinephrine) and ~20% of noradrenaline (norepinephrine) into systemic circulation for systemic effects on multiple organs, and can also send paracrine signals. Hence they are called neuroendocrine cells.

Glomus cells are the cell type mainly located in the carotid bodies and aortic bodies. Glomus type I cells are peripheral chemoreceptors which sense the oxygen, carbon dioxide and pH levels of the blood. When there is a decrease in the blood's pH, a decrease in oxygen (pO2), or an increase in carbon dioxide (pCO2), the carotid bodies and the aortic bodies signal the dorsal respiratory group in the medulla oblongata to increase the volume and rate of breathing. The glomus cells have a high metabolic rate and good blood perfusion and thus are sensitive to changes in arterial blood gas tension. Glomus type II cells are sustentacular cells having a similar supportive function to glial cells.
Autotransplantation is the transplantation of organs, tissues, or even particular proteins from one part of the body to another in the same person.
An amniotic epithelial cell is a form of stem cell extracted from the lining of the inner membrane of the placenta. Amniotic epithelial cells start to develop around 8 days post fertilization. These cells are known to have some of the same markers as embryonic stem cells, more specifically, Oct-4 and nanog. These transcription factors are the basis of the pluripotency of stem cells. Amniotic epithelial cells have the ability to develop into any of the three germ layers: endoderm, mesoderm, and ectoderm. They can develop into several organ tissues specific to these germ layers including heart, brain, and liver. The pluripotency of the human amniotic epithelial cells makes them useful in treating and fighting diseases and disorders of the nervous system as well as other tissues of the human body. Artificial heart valves and working tracheas, as well as muscle, fat, bone, heart, neural and liver cells have all been engineered using amniotic stem cells. Tissues obtained from amniotic cell lines show promise for patients with congenital diseases or malformations of the heart, liver, lungs, kidneys, and cerebral tissue.
In the management of Parkinson's disease, due to the chronic nature of Parkinson's disease (PD), a broad-based program is needed that includes patient and family education, support-group services, general wellness maintenance, exercise, and nutrition. At present, no cure for the disease is known, but medications or surgery can provide relief from the symptoms.
APUD cells (DNES cells) constitute a group of apparently unrelated endocrine cells, which were named by the scientist A.G.E. Pearse, who developed the APUD concept in the 1960s based on calcitonin-secreting parafollicular C cells of dog thyroid. These cells share the common function of secreting a low molecular weight polypeptide hormone. There are several different types which secrete the hormones secretin, cholecystokinin and several others. The name is derived from an acronym, referring to the following:

A paraganglion is a group of non-neuronal cells derived of the neural crest. They are named for being generally in close proximity to sympathetic ganglia. They are essentially of two types: (1) chromaffin or sympathetic paraganglia made of chromaffin cells and (2) nonchromaffin or parasympathetic paraganglia made of glomus cells. They are neuroendocrine cells, the former with primary endocrine functions and the latter with primary chemoreceptor functions.
PC12 is a cell line derived from a pheochromocytoma of the rat adrenal medulla, that have an embryonic origin from the neural crest that has a mixture of neuroblastic cells and eosinophilic cells.

Pigment epithelium-derived factor (PEDF) also known as serpin F1 (SERPINF1), is a multifunctional secreted protein that has anti-angiogenic, anti-tumorigenic, and neurotrophic functions. Found in vertebrates, this 50 kDa protein is being researched as a therapeutic candidate for treatment of such conditions as choroidal neovascularization, heart disease, and cancer. In humans, pigment epithelium-derived factor is encoded by the SERPINF1 gene.

Parkinson's disease (PD), or simply Parkinson's, is a long-term neurodegenerative disease of the central nervous system that affects both the motor system and non-motor systems. The symptoms usually emerge slowly, and as the disease progresses, non-motor symptoms become more common. Usual symptoms are tremor, rigidity, slowness of movement, and difficulty with walking, collectively known as parkinsonism. Parkinson's disease dementia, falls and neuropsychiatric problems such as sleep abnormalities, psychosis, mood swings or behavioral changes may arise in advanced stages.

Ivar Mendez is a neurosurgeon, neuroscientist and Professor of Surgery at the University of Saskatchewan. He is internationally known for his work in cell transplantation for Parkinson's disease and the use of remote presence robotics in neurosurgery and primary health care. In December 2022, Mendez was appointed an officer of the Order of Canada for his pioneering work in the use of remote telemedicine and robotics to revolutionize the delivery of health and patient care.

The sympathoadrenal system is a physiological connection between the sympathetic nervous system and the adrenal medulla and is crucial in an organism's physiological response to outside stimuli. When the body receives sensory information, the sympathetic nervous system sends a signal to preganglionic nerve fibers, which activate the adrenal medulla through acetylcholine. Once activated, norepinephrine and epinephrine are released directly into the blood by adrenomedullary cells where they act as the bodily mechanism for "fight-or-flight" responses. Because of this, the sympathoadrenal system plays a large role in maintaining glucose levels, sodium levels, blood pressure, and various other metabolic pathways that couple with bodily responses to the environment. During numerous diseased states, such as hypoglycemia or even stress, the body's metabolic processes are skewed. The sympathoadrenal system works to return the body to homeostasis through the activation or inactivation of the adrenal gland. However, more severe disorders of the sympathoadrenal system such as pheochromocytoma can affect the body's ability to maintain a homeostatic state. In these cases, curative agents such as adrenergic agonists and antagonists are used to modify epinephrine and norepinephrine levels released by the adrenal medulla.
Gene therapy in Parkinson's disease consists of the creation of new cells that produce a specific neurotransmitter (dopamine), protect the neural system, or the modification of genes that are related to the disease. Then these cells are transplanted to a patient with the disease. There are different kinds of treatments that focus on reducing the symptoms of the disease but currently there is no cure.
Translational neuroscience is the field of study which applies neuroscience research to translate or develop into clinical applications and novel therapies for nervous system disorders. The field encompasses areas such as deep brain stimulation, brain machine interfaces, neurorehabilitation and the development of devices for the sensory nervous system such as the use of auditory implants, retinal implants, and electronic skins.

Anders Björklund' is a Swedish neuroscientist and pioneer in the study of cell- and gene-based reparative and neuroprotective mechanisms in the brain. He has spent his academic career at Lund University in Sweden, as professor since 1983 and as senior professor at the Wallenberg Neuroscience Center since his formal retirement in 2012.
Foetal brain cell graft is a surgical procedure that can be used as a regenerative treatment for various neurological conditions, but was mainly explored and used specifically for treating Parkinson's disease (PD). A standardised procedure is followed: the cells are usually obtained from a 7–8 weeks old foetus and the collected cells undergo testing to examine whether they are free from infectious agents and safe for transplantation. It is found that this procedure results in an overall improvement in motor functions and a reduction in reliance on medication for PD patients.















