
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Cobalt(II) chloride is an inorganic compound, a salt of cobalt and chlorine, with the formula CoCl
2. The compound forms several hydrates CoCl
2·nH
2O, for n = 1, 2, 6, and 9. Claims of the formation of tri- and tetrahydrates have not been confirmed. The anhydrous form is a blue crystalline solid; the dihydrate is purple and the hexahydrate is pink. Commercial samples are usually the hexahydrate, which is one of the most commonly used cobalt salts in the lab.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.
In chemistry, linkage isomerism or ambidentate isomerism is a form of isomerism in which certain coordination compounds have the same composition but differ in their metal atom's connectivity to a ligand.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic solids featuring octahedral Rh(III) centres. Depending on the value of n, the material is either a dense brown solid or a soluble reddish salt. The soluble trihydrated (n = 3) salt is widely used to prepare compounds used in homogeneous catalysis, notably for the industrial production of acetic acid and hydroformylation.

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.

In chemistry, hexol is a cation with formula {[Co(NH3)4(OH)2]3Co}6+ — a coordination complex consisting of four cobalt cations in oxidation state +3, twelve ammonia molecules NH
3, and six hydroxy anions HO−
, with a net charge of +6. The hydroxy groups act as bridges between the central cobalt atom and the other three, which carry the ammonia ligands.

Hexaamminecobalt(III) chloride is the chemical compound with the formula [Co(NH3)6]Cl3. It is the chloride salt of the coordination complex [Co(NH3)6]3+, which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.

Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−. Related salts are also known including Na2PtCl4, which is brown-colored and soluble in alcohols, and quaternary ammonium salts, which are soluble in a broader range of organic solvents.

Tris(ethylenediamine)cobalt(III) chloride is an inorganic compound with the formula [Co(en)3]Cl3 (where "en" is the abbreviation for ethylenediamine). It is the chloride salt of the coordination complex [Co(en)3]3+. This trication was important in the history of coordination chemistry because of its stability and its stereochemistry. Many different salts have been described. The complex was first described by Alfred Werner who isolated this salt as yellow-gold needle-like crystals.
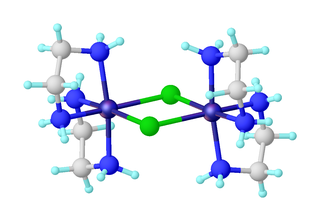
Dichlorobis(ethylenediamine)nickel(II) is the inorganic compound with the formula NiCl2(en)2, where en = ethylenediamine. The formula is deceptive: the compound is the chloride salt of the coordination complex [Ni2Cl2(en)4]2+. This blue solid is soluble in water and some polar organic solvents. It is prepared by ligand redistribution from [Ni(en)3]Cl2 · 2 H2O and hydrated nickel chloride:
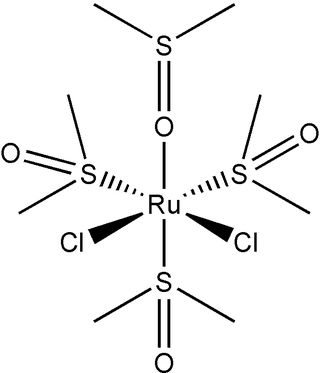
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer is a yellow, air-stable solid that is soluble in some organic solvents. These sulfoxide complexes are used in the synthesis of various ruthenium(ii) complexes. They have also attracted attention as possible anti-cancer drugs.
Cobalt(II) cyanide is the inorganic compound with the formula Co(CN)2. It is coordination polymer that has attracted intermittent attention over many years in the area of inorganic synthesis and homogeneous catalysis.

Chloropentamminecobalt chloride is the dichloride salt of the coordination complex [Co(NH3)5Cl]2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest.
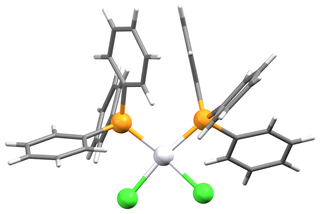
Bis(triphenylphosphine)platinum chloride is a metal phosphine complex with the formula PtCl2[P(C6H5)3]2. Cis- and trans isomers are known. The cis isomer is a white crystalline powder, while the trans isomer is yellow. Both isomers are square planar about the central platinum atom. The cis isomer is used primarily as a reagent for the synthesis of other platinum compounds.

trans-Dichlorobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCl2(en)2]Cl (en = ethylenediamine). It is a green diamagnetic solid that is soluble in water. It is the monochloride salt of the cationic coordination complex [CoCl2(en)2]+. One chloride ion in this salt readily undergoes ion exchange but the two other chlorides are less reactive, being bound to the metal center. The more stable cis-dichlorobis(ethylenediamine)cobalt(III) chloride is also known.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.

Carbonatobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCO3(en)2]Cl (en = ethylenediamine). It is a red diamagnetic solid that is soluble in water. It is the monochloride salt of a cationic carbonate complex [CoCO3(en)2]+. The chloride ion in this salt readily undergoes ion exchange. The compound is synthesized by the oxidation of a mixture of cobalt(II) chloride, lithium hydroxide, and ethylenediamine in the presence of carbon dioxide:
Cobalt compounds are chemical compounds formed by cobalt with other elements.




![UV-vis spectra of various stages in the conversion of trans-[CoCl2(en)2] to the cis isomer. Ultraviolet-visible spectroscopy of Dichlorobis(ethylenediamine)cobalt(III) chloride.png](http://upload.wikimedia.org/wikipedia/commons/thumb/5/5c/Ultraviolet-visible_spectroscopy_of_Dichlorobis%28ethylenediamine%29cobalt%28III%29_chloride.png/220px-Ultraviolet-visible_spectroscopy_of_Dichlorobis%28ethylenediamine%29cobalt%28III%29_chloride.png)
















