Related Research Articles
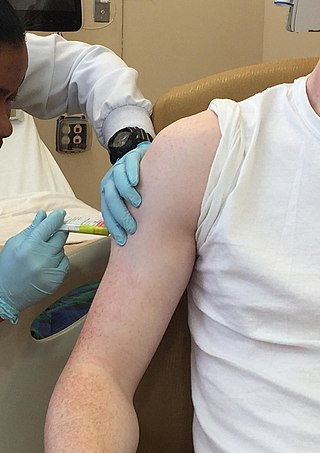
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

In drug development, preclinical development is a stage of research that begins before clinical trials and during which important feasibility, iterative testing and drug safety data are collected, typically in laboratory animals.

The Food and Drug Administration's (FDA) New Drug Application (NDA) is the vehicle in the United States through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing. Some 30% or less of initial drug candidates proceed through the entire multi-year process of drug development, concluding with an approved NDA, if successful.
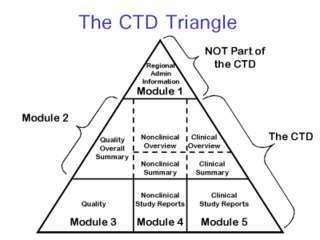
The Common Technical Document (CTD) is a set of specifications for an application dossier for the registration of medicine, designed for use across Europe, Japan, the United States, and beyond.

The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission to start human clinical trials and to ship an experimental drug across state lines before a marketing application for the drug has been approved. Regulations are primarily at 21 CFR 312. Similar procedures are followed in the European Union, Japan, and Canada.
Clinical monitoring is the oversight and administrative efforts that monitor a participant's health and efficacy of the treatment during a clinical trial. Both independent and government-run grant-funding agencies, such as the National Institutes of Health (NIH) and the World Health Organization (WHO), require data and safety monitoring protocols for Phase I and II clinical trials conforming to their standards.
An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign, symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product.

Medical research, also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion of health.
Good clinical practice (GCP) is an international quality standard, which governments can then transpose into regulations for clinical trials involving human subjects. GCP follows the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), and enforces tight guidelines on ethical aspects of clinical research.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.

In health care, a clinical trial is a comparison test of a medication or other medical treatment, versus a placebo, other medications or devices, or the standard medical treatment for a patient's condition.
A glossary of terms used in clinical research.
The following outline is provided as an overview of and topical guide to clinical research:
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Tezepelumab, sold under the brand name Tezspire, is a human monoclonal antibody used for the treatment of asthma. Tezepelumab blocks thymic stromal lymphopoietin (TSLP), an epithelial cytokine that has been suggested to be critical in the initiation and persistence of airway inflammation.

Daprodustat, sold under the brand name Duvroq among others, is a medication that is used for the treatment of anemia due to chronic kidney disease. It is a hypoxia-inducible factor prolyl hydroxylase inhibitor. It is taken by mouth.

Faricimab, sold under the brand name Vabysmo, is a monoclonal antibody used for the treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME). Faricimab is the first bispecific monoclonal antibody to target both vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang-2). By targeting these pathways, faricimab stabilizes blood vessels in the retina. It is given by intravitreal injection by an ophthalmologist.

Sotorasib, sold under the brand names Lumakras and Lumykras, is an anti-cancer medication used to treat non-small-cell lung cancer. It targets a specific mutation, G12C, in the protein K-Ras encoded by gene KRAS which is responsible for various forms of cancer. Sotorasib is an inhibitor of the RAS GTPase family.
Atoltivimab/maftivimab/odesivimab, sold under the brand name Inmazeb, is a fixed-dose combination of three monoclonal antibodies for the treatment of Zaire ebolavirus. It contains atoltivimab, maftivimab, and odesivimab-ebgn and was developed by Regeneron Pharmaceuticals.
References
- 1 2 3 4 5 6 7 8 9 10 "Learn about studies". ClinicalTrials.gov, National Library of Medicine, US National Institutes of Health. 24 May 2023. Retrieved 9 March 2024.
- 1 2 3 4 "What Are Clinical Trials and Studies?". National Institute on Aging, US National Institutes of Health. 22 March 2023. Retrieved 9 March 2024.
- 1 2 3 4 5 6 7 8 "What Are the Different Types of Clinical Research?". US Food and Drug Administration. 4 January 2018. Retrieved 9 March 2024.
- ↑ Mohamadi, Amin; Asghari, Fariba; Rashidian, Arash (2014). "Continuing review of ethics in clinical trials: a surveillance study in Iran". Journal of Medical Ethics and History of Medicine. 7: 22. PMC 4648212 . PMID 26587202.
- ↑ "Public Information Pack (PIP): How to get involved in NHS, public health and social care research". National Institute for Health and Care Research. Retrieved January 3, 2024.
- ↑ "Briefing notes for researchers - public involvement in NHS, health and social care research". National Institute for Health and Care Research. Retrieved January 3, 2024.
- ↑ Ball, Sarah; Harshfield, Amelia; Carpenter, Asha; Bertscher, Adam; Marjanovic, Sonja (2019). Patient and public involvement in research: Enabling meaningful contributions. RAND Corporation. doi:10.7249/rr2678. S2CID 198003937.
- 1 2 3 4 "The Drug Development Process; Step 3: Clinical Research". US Food and Drug Administration. 4 January 2018. Retrieved 28 June 2022.
- ↑ "Authorisation of medicines". European Medicines Agency. 30 October 2023. Retrieved 9 March 2024.