
Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays, usually by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.

Depleted uranium is uranium with a lower content of the fissile isotope 235U than natural uranium. Natural uranium contains about 0.72% 235U, while the DU used by the U.S. Department of Defense contains 0.3% 235U or less. The less radioactive and non-fissile 238U constitutes the main component of depleted uranium.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table. The abundance of an isotope varies from planet to planet, and even from place to place on the Earth, but remains relatively constant in time.
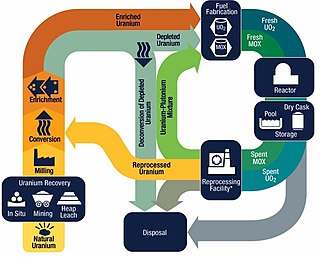
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.
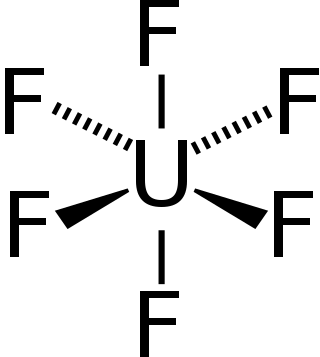
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile and toxic white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

Gaseous diffusion is a technology that was used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through microporous membranes. This produces a slight separation (enrichment factor 1.0043) between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process (enrichment factor 1.05 to 1.2).

Atomic vapor laser isotope separation, or AVLIS, is a method by which specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions. A similar technology, using molecules instead of atoms, is molecular laser isotope separation (MLIS).

The Honeywell Uranium Hexafluoride Processing Facility, a uranium conversion facility, is located 1.9 miles (3 km) northwest of Metropolis, Illinois, United States. The plant, Honeywell Specialty Chemicals in Metropolis, Illinois, has a nominal capacity of 15,000 tU as uranium hexafluoride per year. ConverDyn, a general partnership between affiliates of Honeywell and General Atomics, is the exclusive agent for conversion sales from the Honeywell Uranium Hexafluoride Processing Facility.

Uranium-234 is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% of the raw uranium because its half-life of just 245,500 years is only about 1/18,000 as long as that of 238U. Thus the ratio of 234
U to 238
U in a natural sample is equivalent to the ratio of their half-lives. The primary path of production of 234U via nuclear decay is as follows: uranium-238 nuclei emit an alpha particle to become thorium-234. Next, with a short half-life, 234Th nuclei emit a beta particle to become protactinium-234 (234Pa), or more likely a nuclear isomer denoted 234mPa. Finally, 234Pa or 234mPa nuclei emit another beta particle to become 234U nuclei.
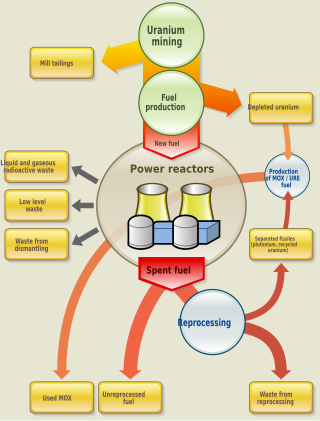
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Uranium (92U) is a naturally occurring radioactive element that has no stable isotope. It has two primordial isotopes, uranium-238 and uranium-235, that have long half-lives and are found in appreciable quantity in the Earth's crust. The decay product uranium-234 is also found. Other isotopes such as uranium-233 have been produced in breeder reactors. In addition to isotopes found in nature or nuclear reactors, many isotopes with far shorter half-lives have been produced, ranging from 214U to 242U. The standard atomic weight of natural uranium is 238.02891(3).

In materials science and materials engineering, uranium metallurgy is the study of the physical and chemical behavior of uranium and its alloys.
Separation of isotopes by laser excitation (SILEX) is a theoretical uranium enrichment technique based on the physical principle of laser condensation repression. The industrial development of this process is mainly managed by the Global Laser Enrichment consortium and the intellectual property so developed is the only private information classified by the US federal government.
Uranium-236 (236U) is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.
Hybrid nuclear fusion–fission is a proposed means of generating power by use of a combination of nuclear fusion and fission processes.
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water (deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for a pressurized water reactor. While heavy water is very expensive to isolate from ordinary water (often referred to as light water in contrast to heavy water), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all current operating reactors.
ConverDyn is a general partnership between American multinational firms General Atomics and Honeywell that provides uranium hexafluoride (UF6) conversion and related services to utilities operating nuclear power plants in North America, Europe, and Asia. The company is the sole marketing agent of UF6 produced at the Honeywell Uranium Hexafluoride Processing Facility in Metropolis, Illinois.












