Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research. By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants, and is also required for the creation of uranium-based nuclear weapons. Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or grade.
Enriched uranium is a type of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Naturally-occurring uranium is composed of three major isotopes: uranium-238, uranium-235, and uranium-234. 235U is the only nuclide existing in nature that is fissile with thermal neutrons.
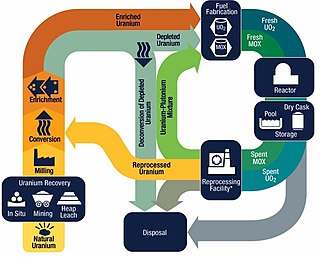
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
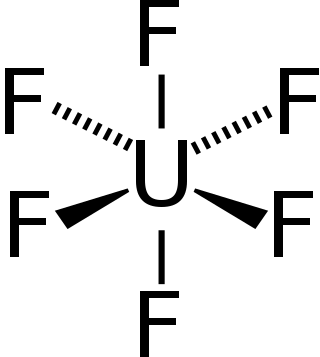
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.

Yellowcake is a type of uranium concentrate powder obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radius of a rotating container. A prominent use of gas centrifuges is for the separation of uranium-235 (235U) from uranium-238 (238U). The gas centrifuge was developed to replace the gaseous diffusion method of uranium-235 extraction. High degrees of separation of these isotopes relies on using many individual centrifuges arranged in series, that achieve successively higher concentrations. This process yields higher concentrations of uranium-235 while using significantly less energy compared to the gaseous diffusion process.
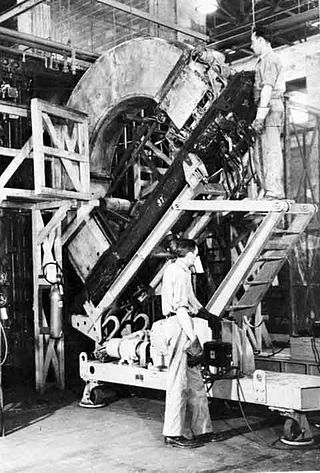
A calutron is a mass spectrometer originally designed and used for separating the isotopes of uranium. It was developed by Ernest Lawrence during the Manhattan Project and was based on his earlier invention, the cyclotron. Its name was derived from California University Cyclotron, in tribute to Lawrence's institution, the University of California, where it was invented. Calutrons were used in the industrial-scale Y-12 uranium enrichment plant at the Clinton Engineer Works in Oak Ridge, Tennessee. The enriched uranium produced was used in the Little Boy atomic bomb that was detonated over Hiroshima on 6 August 1945.
Erich Rudolf Bagge was a German scientist. Bagge, a student of Werner Heisenberg for his doctorate and Habilitation, was engaged in German Atomic Energy research and the German nuclear energy project during the Second World War. He worked as an Assistant at the Kaiser-Wilhelm-Institut für Physik in Berlin. Bagge, who became associated professor at the University of Hamburg in 1948, was in particular involved in the usage of nuclear power for trading vessels, and he was one of the founders of the Society for the Usage of Nuclear Energy in Ship-Building and Seafare.

Atomic vapor laser isotope separation, or AVLIS, is a method by which specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions. A similar technology, using molecules instead of atoms, is molecular laser isotope separation (MLIS).

K-25 was the codename given by the Manhattan Project to the program to produce enriched uranium for atomic bombs using the gaseous diffusion method. Originally the codename for the product, over time it came to refer to the project, the production facility located at the Clinton Engineer Works in Oak Ridge, Tennessee, the main gaseous diffusion building, and ultimately the site. When it was built in 1944, the four-story K-25 gaseous diffusion plant was the world's largest building, comprising over 5,264,000 square feet (489,000 m2) of floor space and a volume of 97,500,000 cubic feet (2,760,000 m3).

Eurodif, which means European Gaseous Diffusion Uranium Enrichment Consortium, is a subsidiary of the French company Orano, which operates a uranium enrichment plant established at the Tricastin Nuclear Power Center in Pierrelatte in Drôme. The nuclear site of Pierrelatte includes many nuclear installations, of which the largest are the Eurodif fuel factory and the Tricastin nuclear power station.
Molecular laser isotope separation (MLIS) is a method of isotope separation, where specially tuned lasers are used to separate isotopes of uranium using selective ionization of hyperfine transitions of uranium hexafluoride molecules. It is similar to AVLIS. Its main advantage over AVLIS is low energy consumption and use of uranium hexafluoride instead of vaporized uranium.

Uranium tetrachloride is an inorganic compound, a salt of uranium and chlorine, with the formula UCl4. It is a hygroscopic olive-green solid. It was used in the electromagnetic isotope separation (EMIS) process of uranium enrichment. It is one of the main starting materials for organouranium chemistry.
Separation of isotopes by laser excitation (SILEX) is a process under development to enrich uranium on an industrial scale for nuclear reactors. It is strongly suspected that it utilizes laser condensation repression to excite the uranium-235 isotope in uranium hexafluoride (UF6), allowing this lighter molecule to move more rapidly to the outer rim of a gaseous jet and resist condensing compared to the heavier, unexcited 238UF6. This differs greatly from previous methods of laser enrichment explored for their commercial prospects: one using atomic uranium (Atomic Vapor Laser Isotope Separation (AVLIS)) and another molecular method that uses lasers to dissociate a fluorine atom from 235UF6 (Molecular Laser Isotope Separation (MLIS)), allowing the enriched product to precipitate out as a solid.

The Portsmouth Gaseous Diffusion Plant is a facility located in Scioto Township, Pike County, Ohio, just south of Piketon, Ohio, that previously produced enriched uranium, including highly enriched weapons-grade uranium, for the United States Atomic Energy Commission (AEC), the U.S. nuclear weapons program and Navy nuclear propulsion; in later years, it produced low-enriched uranium for fuel for commercial nuclear power reactors. The site never hosted an operating nuclear reactor.
The Zippe-type centrifuge is a gas centrifuge designed to enrich the rare fissile isotope uranium-235 (235U) from the mixture of isotopes found in naturally occurring uranium compounds. The isotopic separation is based on the slight difference in mass of the isotopes. The Zippe design was originally developed in the Soviet Union by a team led by 60 Austrian and German scientists and engineers captured after World War II, working in detention. In the West the type is known by the name of the man who recreated the technology after his return to the West in 1956, based on his recollection of his work in the Soviet program, Gernot Zippe. To the extent that it might be referred to in Soviet/Russian usage by any one person's name, it was known as a Kamenev centrifuge.

The Paducah Gaseous Diffusion Plant (PGDP) is a facility located in McCracken County, Kentucky, near Paducah, Kentucky that produced enriched uranium from 1952 to 2013. It is owned by the U.S. Department of Energy (DOE). The PGDP was the only operating uranium enrichment facility in the United States from 2001 to 2010. The Paducah plant produced low-enriched uranium, originally as feedstock for military reactors and weapons, and later for commercial nuclear power fuel.
William Taylor Miller was an American professor of organic chemistry at Cornell University. His experimental research included investigations into the mechanism of addition of halogens, especially fluorine, to hydrocarbons. His work focused primarily on the physical and chemical properties of fluorocarbons and chlorofluorocarbons, and the synthesis of novel electrophilic reagents.
ConverDyn is a general partnership between American multinational firms General Atomics and Honeywell that provides uranium hexafluoride (UF6) conversion and related services to utilities operating nuclear power plants in North America, Europe, and Asia. The company is the sole marketing agent of UF6 produced at the Honeywell Uranium Hexafluoride Processing Facility in Metropolis, Illinois.
Depleted uranium hexafluoride (DUHF; also referred to as depleted uranium tails, depleted uranium tailings or DUF6) is a byproduct of the processing of uranium hexafluoride into enriched uranium. It is one of the chemical forms of depleted uranium (up to 73-75%), along with depleted triuranium octoxide (up to 25%) and depleted uranium metal (up to 2%). DUHF is 1.7 times less radioactive than uranium hexafluoride and natural uranium.












