Related Research Articles
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactive decay.

Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
In nuclear engineering, fissile material is material that can undergo nuclear fission when struck by a neutron of low energy. A self-sustaining thermal chain reaction can only be achieved with fissile material. The predominant neutron energy in a system may be typified by either slow neutrons or fast neutrons. Fissile material can be used to fuel thermal-neutron reactors, fast-neutron reactors and nuclear explosives.
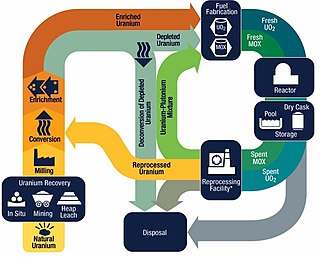
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the light-water reactors that predominate nuclear power generation.

A breeder reactor is a nuclear reactor that generates more fissile material than it consumes. These reactors can be fueled with more-commonly available isotopes of uranium and thorium, such as uranium-238 and thorium-232, as opposed to the rare uranium-235 which is used in conventional reactors. These materials are called fertile materials since they can be bred into fuel by these breeder reactors.

Uranium-238 is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.

Uranium-234 is an isotope of uranium. In natural uranium and in uranium ore, 234U occurs as an indirect decay product of uranium-238, but it makes up only 0.0055% of the raw uranium because its half-life of just 245,500 years is only about 1/18,000 as long as that of 238U. Thus the ratio of 234
U to 238
U in a natural sample is equivalent to the ratio of their half-lives. The primary path of production of 234U via nuclear decay is as follows: uranium-238 nuclei emit an alpha particle to become thorium-234. Next, with a short half-life, 234Th nuclei emit a beta particle to become protactinium-234 (234Pa), or more likely a nuclear isomer denoted 234mPa. Finally, 234Pa or 234mPa nuclei emit another beta particle to become 234U nuclei.
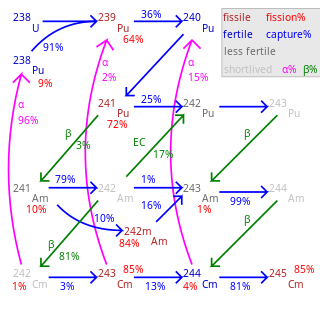
Fertile material is a material that, although not fissile itself, can be converted into a fissile material by neutron absorption.
Thorium-232 is the main naturally occurring isotope of thorium, with a relative abundance of 99.98%. It has a half life of 14 billion years, which makes it the longest-lived isotope of thorium. It decays by alpha decay to radium-228; its decay chain terminates at stable lead-208.
Uranium-233 is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in experimental nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years.

Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in thermal spectrum nuclear reactors, along with uranium-235 and uranium-233. Plutonium-239 has a half-life of 24,110 years.
Protactinium (91Pa) has no stable isotopes. The four naturally occurring isotopes allow a standard atomic weight to be given.
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding 238
U
with neutrons to produce 239
U
, which then underwent beta decay to 239
Np
.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years, plutonium-242 with a half-life of 373,300 years, and plutonium-239 with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states; all have half-lives of less than one second.

The thorium fuel cycle is a nuclear fuel cycle that uses an isotope of thorium, 232
Th
, as the fertile material. In the reactor, 232
Th
is transmuted into the fissile artificial uranium isotope 233
U
which is the nuclear fuel. Unlike natural uranium, natural thorium contains only trace amounts of fissile material, which are insufficient to initiate a nuclear chain reaction. Additional fissile material or another neutron source is necessary to initiate the fuel cycle. In a thorium-fuelled reactor, 232
Th
absorbs neutrons to produce 233
U
. This parallels the process in uranium breeder reactors whereby fertile 238
U
absorbs neutrons to form fissile 239
Pu
. Depending on the design of the reactor and fuel cycle, the generated 233
U
either fissions in situ or is chemically separated from the used nuclear fuel and formed into new nuclear fuel.

Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor. It is no longer useful in sustaining a nuclear reaction in an ordinary thermal reactor and, depending on its point along the nuclear fuel cycle, it will have different isotopic constituents than when it started.
Uranium-236 (236U) is an isotope of uranium that is neither fissile with thermal neutrons, nor very good fertile material, but is generally considered a nuisance and long-lived radioactive waste. It is found in spent nuclear fuel and in the reprocessed uranium made from spent nuclear fuel.

Nuclear transmutation is the conversion of one chemical element or an isotope into another chemical element. Nuclear transmutation occurs in any process where the number of protons or neutrons in the nucleus of an atom is changed.
References
- 1 2 3 4 5 Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ↑ Magurno, B.A.; Pearlstein, S, eds. (1981). Proceedings of the conference on nuclear data evaluation methods and procedures. BNL-NCS 51363, vol. II (PDF). Upton, NY (USA): Brookhaven National Lab. pp. 835 ff. Retrieved 2014-08-06.
- ↑ "Standard Atomic Weights: Uranium". CIAAW. 1999.
- ↑ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; et al. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ↑ "Uranium Isotopes". GlobalSecurity.org. Retrieved 14 March 2012.
- ↑ Wang, Meng; Huang, W.J.; Kondev, F.G.; Audi, G.; Naimi, S. (2021). "The AME 2020 atomic mass evaluation (II). Tables, graphs and references*". Chinese Physics C. 45 (3): 030003. doi:10.1088/1674-1137/abddaf.
- ↑ Zhang, Z. Y.; Yang, H. B.; Huang, M. H.; Gan, Z. G.; Yuan, C. X.; Qi, C.; Andreyev, A. N.; Liu, M. L.; Ma, L.; Zhang, M. M.; Tian, Y. L.; Wang, Y. S.; Wang, J. G.; Yang, C. L.; Li, G. S.; Qiang, Y. H.; Yang, W. Q.; Chen, R. F.; Zhang, H. B.; Lu, Z. W.; Xu, X. X.; Duan, L. M.; Yang, H. R.; Huang, W. X.; Liu, Z.; Zhou, X. H.; Zhang, Y. H.; Xu, H. S.; Wang, N.; Zhou, H. B.; Wen, X. J.; Huang, S.; Hua, W.; Zhu, L.; Wang, X.; Mao, Y. C.; He, X. T.; Wang, S. Y.; Xu, W. Z.; Li, H. W.; Ren, Z. Z.; Zhou, S. G. (2021). "New α-Emitting Isotope U214 and Abnormal Enhancement of α-Particle Clustering in Lightest Uranium Isotopes". Physical Review Letters. 126 (15): 152502. arXiv: 2101.06023 . Bibcode:2021PhRvL.126o2502Z. doi:10.1103/PhysRevLett.126.152502. PMID 33929212. S2CID 231627674.
- 1 2 Zhang, M. M.; Tian, Y. L.; Wang, Y. S.; Zhang, Z. Y.; Gan, Z. G.; Yang, H. B.; Huang, M. H.; Ma, L.; Yang, C. L.; Wang, J. G.; Yuan, C. X.; Qi, C.; Andreyev, A. N.; Huang, X. Y.; Xu, S. Y.; Zhao, Z.; Chen, L. X.; Wang, J. Y.; Liu, M. L.; Qiang, Y. H.; Li, G. S.; Yang, W. Q.; Chen, R. F.; Zhang, H. B.; Lu, Z. W.; Xu, X. X.; Duan, L. M.; Yang, H. R.; Huang, W. X.; Liu, Z.; Zhou, X. H.; Zhang, Y. H.; Xu, H. S.; Wang, N.; Zhou, H. B.; Wen, X. J.; Huang, S.; Hua, W.; Zhu, L.; Wang, X.; Mao, Y. C.; He, X. T.; Wang, S. Y.; Xu, W. Z.; Li, H. W.; Niu, Y. F.; Guo, L.; Ren, Z. Z.; Zhou, S. G. (4 August 2022). "Fine structure in the α decay of the 8+ isomer in 216, 218U". Physical Review C. 106 (2): 024305. doi:10.1103/PhysRevC.106.024305. ISSN 2469-9985. S2CID 251359451.
- ↑ Gan, ZaiGuo; Jiang, Jian; Yang, HuaBin; Zhang, ZhiYuan; Ma, Long; Yu, Lin; Wang, JianGuo; Tian, YuLin; Ding, Bing; Huang, TianHeng; Wang, YongSheng; Guo, Song; Sun, MingDao; Wang, KaiLong; Zhou, ShanGui; Ren, ZhongZhou; Zhou, XiaoHong; Xu, HuShan (1 August 2016). "α decay studies of the neutron-deficient uranium isotopes 215-217U". Chinese Science Bulletin. 61 (22): 2502–2511. doi: 10.1360/N972015-01316 . Retrieved 24 June 2023.
- ↑ Trenn, Thaddeus J. (1978). "Thoruranium (U-236) as the extinct natural parent of thorium: The premature falsification of an essentially correct theory". Annals of Science. 35 (6): 581–97. doi:10.1080/00033797800200441.
- 1 2 Bonetti, R.; Guglielmetti, A. (2007). "Cluster radioactivity: an overview after twenty years" (PDF). Romanian Reports in Physics. 59: 301–310. Archived from the original (PDF) on 19 September 2016.
- ↑ Niwase, T.; Watanabe, Y. X.; Hirayama, Y.; et al. (2023). "Discovery of New Isotope 241U and Systematic High-Precision Atomic Mass Measurements of Neutron-Rich Pa-Pu Nuclei Produced via Multinucleon Transfer Reactions" (PDF). Physical Review Letters. 130 (13): 132502-1–132502-6. doi:10.1103/PhysRevLett.130.132502. PMID 37067317. S2CID 257976576.
- 1 2 Mukunth, Vasudevan (2023-04-05). "In pursuit of a 'magic number', physicists discover new uranium isotope". The Hindu . ISSN 0971-751X . Retrieved 2023-04-12.
- ↑ Yirka, Bob (April 5, 2023). "Previously unknown isotope of uranium discovered". Phys.org . Retrieved 2023-04-12.
- ↑ Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three-element gap of instability after polonium (84) where no nuclides have half-lives of at least four years (the longest-lived nuclide in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at 1,600 years, thus merits the element's inclusion here.
- ↑ Specifically from thermal neutron fission of uranium-235, e.g. in a typical nuclear reactor.
- ↑ Milsted, J.; Friedman, A. M.; Stevens, C. M. (1965). "The alpha half-life of berkelium-247; a new long-lived isomer of berkelium-248". Nuclear Physics. 71 (2): 299. Bibcode:1965NucPh..71..299M. doi:10.1016/0029-5582(65)90719-4.
"The isotopic analyses disclosed a species of mass 248 in constant abundance in three samples analysed over a period of about 10 months. This was ascribed to an isomer of Bk248 with a half-life greater than 9 [years]. No growth of Cf248 was detected, and a lower limit for the β− half-life can be set at about 104 [years]. No alpha activity attributable to the new isomer has been detected; the alpha half-life is probably greater than 300 [years]." - ↑ This is the heaviest nuclide with a half-life of at least four years before the "sea of instability".
- ↑ Excluding those "classically stable" nuclides with half-lives significantly in excess of 232Th; e.g., while 113mCd has a half-life of only fourteen years, that of 113Cd is nearly eight quadrillion years.
- ↑ "Physicists Discover New Uranium Isotope: Uranium-214". Sci-News.com. 14 May 2021. Retrieved 15 May 2021.
- ↑ Zhang, Z. Y.; et al. (2021). "New α -Emitting Isotope 214 U and Abnormal Enhancement of α -Particle Clustering in Lightest Uranium Isotopes". Physical Review Letters. 126 (15): 152502. arXiv: 2101.06023 . Bibcode:2021PhRvL.126o2502Z. doi:10.1103/PhysRevLett.126.152502. PMID 33929212. S2CID 231627674 . Retrieved 15 May 2021.
- ↑ "Lightest-known form of uranium created". Live Science. 3 May 2021. Retrieved 15 May 2021.
- ↑ "Physicists have created a new and extremely rare kind of uranium". New Scientist. Retrieved 15 May 2021.
- ↑ "Uranium 232". Nuclear Power. Archived from the original on 26 February 2019. Retrieved 3 June 2019.
- ↑ "INCIDENT NEUTRON DATA". atom.kaeri.re.kr. 2011-12-14.
- ↑ C. W. Forsburg; L. C. Lewis (1999-09-24). "Uses For Uranium-233: What Should Be Kept for Future Needs?" (PDF). Ornl-6952. Oak Ridge National Laboratory.
- ↑ Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- 1 2 Ronen, Y., ed. (1990). High converting water reactors. CRC Press. p. 212. ISBN 0-8493-6081-1. LCCN 89-25332.
- 1 2 Use of Reprocessed Uranium (PDF). Technical Document. Vienna: International Atomic Energy Agency. 2009. ISBN 978-92-0-157109-0. ISSN 1684-2073.
- ↑ B. C. Diven; J. Terrell; A. Hemmendinger (1 January 1958). "Capture-to-Fission Ratios for Fast Neutrons in U235". Physical Review Letters. 109 (1): 144–150. Bibcode:1958PhRv..109..144D. doi:10.1103/PhysRev.109.144.
- ↑ Ikeda, Nagao (July 25, 2011). "The discoveries of uranium 237 and symmetric fission — From the archival papers of Nishina and Kimura". Proceedings of the Japan Academy. Series B, Physical and Biological Sciences. 87 (7): 371–376. doi:10.2183/pjab.87.371. PMC 3171289 . PMID 21785255.
- ↑ CRC Handbook of Chemistry and Physics, 57th Ed. p. B-345
- ↑ CRC Handbook of Chemistry and Physics, 57th Ed. p. B-423
- 1 2 Yirka, Bob; Phys.org. "Previously unknown isotope of uranium discovered". phys.org. Retrieved 2023-04-10.
- ↑ Niwase, T.; Watanabe, Y. X.; Hirayama, Y.; Mukai, M.; Schury, P.; Andreyev, A. N.; Hashimoto, T.; Iimura, S.; Ishiyama, H.; Ito, Y.; Jeong, S. C.; Kaji, D.; Kimura, S.; Miyatake, H.; Morimoto, K. (2023-03-31). "Discovery of New Isotope $^{241}\mathrm{U}$ and Systematic High-Precision Atomic Mass Measurements of Neutron-Rich Pa-Pu Nuclei Produced via Multinucleon Transfer Reactions". Physical Review Letters. 130 (13): 132502. doi:10.1103/PhysRevLett.130.132502. PMID 37067317. S2CID 257976576.