Fluorine (9F) has 18 known isotopes ranging from 13
F
to 31
F
and two isomers. Only fluorine-19 is stable and naturally occurring in more than trace quantities; therefore, fluorine is a monoisotopic and mononuclidic element.
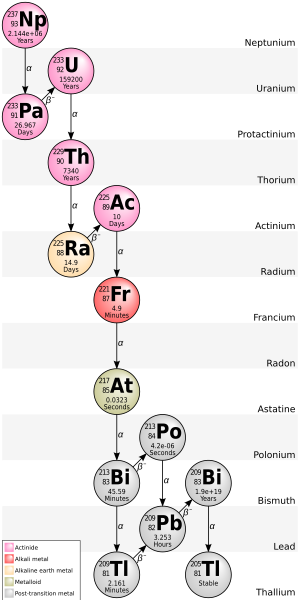
Francium (87Fr) has no stable isotopes. A standard atomic weight cannot be given. Its most stable isotope is 223Fr with a half-life of 22 minutes, occurring in trace quantities in nature as an intermediate decay product of 235U.
Astatine (85At) has 41 known isotopes, all of which are radioactive; their mass numbers range from 188 to 229. There are also 24 known metastable excited states. The longest-lived isotope is 210At, which has a half-life of 8.1 hours; the longest-lived isotope existing in naturally occurring decay chains is 219At with a half-life of 56 seconds.
There are 42 isotopes of polonium (84Po). They range in size from 186 to 227 nucleons. They are all radioactive. 210Po with a half-life of 138.376 days has the longest half-life of any naturally-occurring isotope of polonium and is the most common isotope of polonium. It is also the most easily synthesized polonium isotope. 209Po, which does not occur naturally, has the longest half-life of all isotopes of polonium at 125.2 years. 209Po can be made by using a cyclotron to bombard bismuth with protons, as can 208Po.
Bismuth (83Bi) has 41 known isotopes, ranging from 184Bi to 224Bi. Bismuth has no stable isotopes, but does have one very long-lived isotope; thus, the standard atomic weight can be given as 208.98040(1). Although bismuth-209 is now known to be radioactive, it has classically been considered to be a stable isotope because it has a half-life of approximately 2.01×1019 years, which is more than a billion times the age of the universe. Besides 209Bi, the most stable bismuth radioisotopes are 210mBi with a half-life of 3.04 million years, 208Bi with a half-life of 368,000 years and 207Bi, with a half-life of 32.9 years, none of which occurs in nature. All other isotopes have half-lives under 1 year, most under a day. Of naturally occurring radioisotopes, the most stable is radiogenic 210Bi with a half-life of 5.012 days. 210mBi is unusual for being a nuclear isomer with a half-life multiple orders of magnitude longer than that of the ground state.
There are seven stable isotopes of mercury (80Hg) with 202Hg being the most abundant (29.86%). The longest-lived radioisotopes are 194Hg with a half-life of 444 years, and 203Hg with a half-life of 46.612 days. Most of the remaining 40 radioisotopes have half-lives that are less than a day. 199Hg and 201Hg are the most often studied NMR-active nuclei, having spin quantum numbers of 1/2 and 3/2 respectively. All isotopes of mercury are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed. These isotopes are predicted to undergo either alpha decay or double beta decay.
Naturally occurring platinum (78Pt) consists of five stable isotopes (192Pt, 194Pt, 195Pt, 196Pt, 198Pt) and one very long-lived (half-life 6.50×1011 years) radioisotope (190Pt). There are also 34 known synthetic radioisotopes, the longest-lived of which is 193Pt with a half-life of 50 years. All other isotopes have half-lives under a year, most under a day. All isotopes of platinum are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed. Platinum-195 is the most abundant isotope.
There are two natural isotopes of iridium (77Ir), and 37 radioisotopes, the most stable radioisotope being 192Ir with a half-life of 73.83 days, and many nuclear isomers, the most stable of which is 192m2Ir with a half-life of 241 years. All other isomers have half-lives under a year, most under a day. All isotopes of iridium are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed.
Naturally occurring erbium (68Er) is composed of 6 stable isotopes, with 166Er being the most abundant. 39 radioisotopes have been characterized with between 74 and 112 neutrons, or 142 to 180 nucleons, with the most stable being 169Er with a half-life of 9.4 days, 172Er with a half-life of 49.3 hours, 160Er with a half-life of 28.58 hours, 165Er with a half-life of 10.36 hours, and 171Er with a half-life of 7.516 hours. All of the remaining radioactive isotopes have half-lives that are less than 3.5 hours, and the majority of these have half-lives that are less than 4 minutes. This element also has numerous meta states, with the most stable being 167mEr.
Naturally occurring praseodymium (59Pr) is composed of one stable isotope, 141Pr. Thirty-eight radioisotopes have been characterized with the most stable being 143Pr, with a half-life of 13.57 days and 142Pr, with a half-life of 19.12 hours. All of the remaining radioactive isotopes have half-lives that are less than 5.985 hours and the majority of these have half-lives that are less than 33 seconds. This element also has 15 meta states with the most stable being 138mPr, 142mPr and 134mPr.
Naturally occurring silver (47Ag) is composed of the two stable isotopes 107Ag and 109Ag in almost equal proportions, with 107Ag being slightly more abundant. Notably, silver is the only element with all stable istopes having nuclear spins of 1/2. Thus both 107Ag and 109Ag nuclei produce narrow lines in nuclear magnetic resonance spectra.
Bromine (35Br) has two stable isotopes, 79Br and 81Br, and 32 known radioisotopes, the most stable of which is 77Br, with a half-life of 57.036 hours.
Calcium (20Ca) has 26 known isotopes, ranging from 35Ca to 60Ca. There are five stable isotopes, plus one isotope (48Ca) with such a long half-life that for all practical purposes it can be considered stable. The most abundant isotope, 40Ca, as well as the rare 46Ca, are theoretically unstable on energetic grounds, but their decay has not been observed. Calcium also has a cosmogenic isotope, radioactive 41Ca, which has a half-life of 99,400 years. Unlike cosmogenic isotopes that are produced in the atmosphere, 41Ca is produced by neutron activation of 40Ca. Most of its production is in the upper metre of the soil column, where the cosmogenic neutron flux is still sufficiently strong. 41Ca has received much attention in stellar studies because it decays to 41K, a critical indicator of solar system anomalies. The most stable artificial radioisotopes are 45Ca with a half-life of 163 days and 47Ca with a half-life of 4.5 days. All other calcium isotopes have half-lives measured in minutes or less.

Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. 28Si, 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray spallation of argon. Its half-life has been determined to be approximately 150 years, and it decays by beta emission to 32P and then to 32S. After 32Si, 31Si has the second longest half-life at 157.3 minutes. All others have half-lives under 7 seconds.

Magnesium (12Mg) naturally occurs in three stable isotopes: 24
Mg, 25
Mg, and 26
Mg. There are 19 radioisotopes that have been discovered, ranging from 18
Mg to 40
Mg. The longest-lived radioisotope is 28
Mg with a half-life of 20.915(9) h. The lighter isotopes mostly decay to isotopes of sodium while the heavier isotopes decay to isotopes of aluminium. The shortest-lived is proton-unbound 19
Mg with a half-life of 5(3) picoseconds, though the half-life of similarly unbound 18
Mg has not been measured.
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95. Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
Californium (98Cf) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 245Cf in 1950. There are 20 known radioisotopes ranging from 237Cf to 256Cf and one nuclear isomer, 249mCf. The longest-lived isotope is 251Cf with a half-life of 898 years.
Einsteinium (99Es) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be discovered was 253Es in 1952. There are 18 known radioisotopes from 240Es to 257Es, and 3 nuclear isomers. The longest-lived isotope is 252Es with a half-life of 471.7 days, or around 1.293 years.
Mendelevium (101Md) is a synthetic element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 256Md in 1955. There are 17 known radioisotopes, ranging in atomic mass from 244Md to 260Md, and 5 isomers. The longest-lived isotope is 258Md with a half-life of 51.3 days, and the longest-lived isomer is 258mMd with a half-life of 57 minutes.
Lawrencium (103Lr) is a synthetic element, and thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 258Lr in 1961. There are fourteen known isotopes from 251Lr to 266Lr, and seven isomers. The longest-lived known isotope is 266Lr with a half-life of 11 hours.