Notable isotopes
This section needs additional citations for verification .(May 2018) |
This section possibly contains original research .(May 2018) |
The 5 stable and 30 unstable isotopes of nickel range in atomic weight from 48
Ni
to 82
Ni
, and include: [6]
Nickel-48, discovered in 1999, is the most neutron-poor nickel isotope known. With 28 protons and 20 neutrons 48
Ni
is "doubly magic" (like 208
Pb
) and therefore much more stable (with a lower limit of its half-life-time of .5 μs) than would be expected from its position in the chart of nuclides. [7] It has the highest ratio of protons to neutrons (proton excess) of any known doubly magic nuclide. [8]
Nickel-56 is produced in large quantities in supernovae. In the last phases of stellar evolution of very large stars, nuclear fusion of lighter elements like hydrogen and helium comes to an end. Later in the star’s life cycle, elements including magnesium, silicon, and sulfur are fused to form heavier elements. Once the last nuclear fusion reactions cease, the star collapses to produce a supernova. During the supernova, silicon burning produces 56Ni. This isotope of nickel is favored because it has an equal number of neutrons and protons, making it readily produced by fusing two 28Si atoms. 56Ni is the final element that can be formed in the alpha process. Past 56Ni, nuclear reactions would be endoergic and would be energetically unfavorable. Once 56Ni is formed it subsequently decays to 56Co and then 56Fe. [9] The radioactive decay of 56Ni and 56Co supplies much of the energy for the light curves observed for stellar supernovae. [10] The shape of the light curve of these supernovae display characteristic timescales corresponding to the decay of 56Ni to 56Co and then to 56 Fe.
Nickel-58 is the most abundant isotope of nickel, making up 68.077% of the natural abundance. Possible sources include electron capture from copper-58 and EC + p from zinc-59.
Nickel-59 is a long-lived cosmogenic radionuclide with a half-life of 76,000 years. 59
Ni
has found many applications in isotope geology. 59
Ni
has been used to date the terrestrial age of meteorites and to determine abundances of extraterrestrial dust in ice and sediment.
Nickel-60 is the daughter product of the extinct radionuclide 60
Fe
(half-life = 2.6 My). Because 60
Fe
had such a long half-life, its persistence in materials in the Solar System at high enough concentrations may have generated observable variations in the isotopic composition of 60
Ni
. Therefore, the abundance of 60
Ni
present in extraterrestrial material may provide insight into the origin of the Solar System and its early history/very early history. Unfortunately, nickel isotopes appear to have been heterogeneously distributed in the early Solar System. Therefore, so far, no actual age information has been attained from 60
Ni
excesses. 60
Ni
is also the stable end-product of the decay of 60
Zn
, the product of the final rung of the alpha ladder. Other sources may also include beta decay from cobalt-60 and electron capture from copper-60.
Nickel-61 is the only stable isotope of nickel with a nuclear spin (I = 3/2), which makes it useful for studies by EPR spectroscopy. [11]
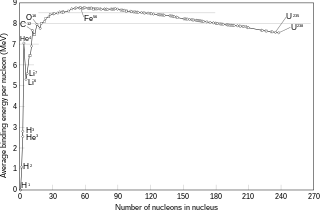
Nickel-62 has the highest binding energy per nucleon of any isotope for any element, when including the electron shell in the calculation. More energy is released forming this isotope than any other, although fusion can form heavier isotopes. For instance, two 40
Ca
atoms can fuse to form 80
Kr
plus 4 positrons (plus 4 neutrinos), liberating 77 keV per nucleon, but reactions leading to the iron/nickel region are more probable as they release more energy per baryon.
Nickel-63 has two main uses: Detection of explosives traces, and in certain kinds of electronic devices, such as gas discharge tubes used as surge protectors. A surge protector is a device that protects sensitive electronic equipment like computers from sudden changes in the electric current flowing into them. It is also used in Electron capture detector in gas chromatography for the detection mainly of halogens. It is proposed to be used for miniature betavoltaic generators for pacemakers.
Nickel-64 is another stable isotope of nickel. Possible sources include beta decay from cobalt-64, and electron capture from copper-64.
Nickel-78 is one of the element's heaviest known isotopes. With 28 protons and 50 neutrons, nickel-78 is doubly magic, resulting in much greater nuclear binding energy and stability despite having a lopsided neutron-proton ratio. It has a half-life of 122 ± 5.1 milliseconds. [12] As a consequence of its magic neutron number, nickel-78 is believed to have an important involvement in supernova nucleosynthesis of elements heavier than iron. [13] 78Ni, along with N = 50 isotones 79Cu and 80Zn, are thought to constitute a waiting point in the r-process, where further neutron capture is delayed by the shell gap and a buildup of isotopes around A = 80 results. [14]