
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

Single-photon emission computed tomography is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera, but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required.

Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitted from within the body rather than radiation that is transmitted through the body from external sources like X-ray generators. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.

Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by gamma cameras, which are external detectors that form two-dimensional images in a process similar to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images and are therefore classified as separate techniques from scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
Natural gallium (31Ga) consists of a mixture of two stable isotopes: gallium-69 and gallium-71. Twenty-nine radioisotopes are known, all synthetic, with atomic masses ranging from 56 to 86; along with three nuclear isomers, 64mGa, 72mGa and 74mGa. Most of the isotopes with atomic mass numbers below 69 decay to isotopes of zinc, while most of the isotopes with masses above 71 decay to isotopes of germanium. Among them, the most commercially important radioisotopes are gallium-67 and gallium-68.
Copper (29Cu) has two stable isotopes, 63Cu and 65Cu, along with 27 radioisotopes. The most stable radioisotope is 67Cu with a half-life of 61.83 hours, while the least stable is 54Cu with a half-life of approximately 75 ns. Most have half-lives under a minute. Unstable copper isotopes with atomic masses below 63 tend to undergo β+ decay, while isotopes with atomic masses above 65 tend to undergo β− decay. 64Cu decays by both β+ and β−.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.
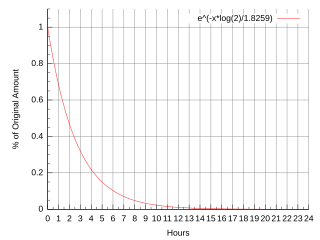
Fluorine-18 (18F) is a fluorine radioisotope which is an important source of positrons. It has a mass of 18.0009380(6) u and its half-life is 109.771(20) minutes. It decays by positron emission 96.7% of the time and electron capture 3.3% of the time. Both modes of decay yield stable oxygen-18.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).

An octreotide scan is a type of SPECT scintigraphy used to find carcinoid, pancreatic neuroendocrine tumors, and to localize sarcoidosis. It is also called somatostatin receptor scintigraphy (SRS). Octreotide, a drug similar to somatostatin, is radiolabeled with indium-111, and is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin. A gamma camera detects the radioactive octreotide, and makes pictures showing where the tumor cells are in the body, typically by a SPECT technique. A technetium-99m based radiopharmaceutical kit is also available.
Nuclear medicine physicians, also called nuclear radiologists or simply nucleologists, are medical specialists that use tracers, usually radiopharmaceuticals, for diagnosis and therapy. Nuclear medicine procedures are the major clinical applications of molecular imaging and molecular therapy. In the United States, nuclear medicine physicians are certified by the American Board of Nuclear Medicine and the American Osteopathic Board of Nuclear Medicine.
Scandium-44 (44Sc) is a radioactive isotope of scandium that decays by positron emission to stable 44Ca with a half-life of 4.042 hours.
Preclinical imaging is the visualization of living animals for research purposes, such as drug development. Imaging modalities have long been crucial to the researcher in observing changes, either at the organ, tissue, cell, or molecular level, in animals responding to physiological or environmental changes. Imaging modalities that are non-invasive and in vivo have become especially important to study animal models longitudinally. Broadly speaking, these imaging systems can be categorized into primarily morphological/anatomical and primarily molecular imaging techniques. Techniques such as high-frequency micro-ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) are usually used for anatomical imaging, while optical imaging, positron emission tomography (PET), and single photon emission computed tomography (SPECT) are usually used for molecular visualizations.

DOTA-TATE is an eight amino acid long peptide, with a covalently bonded DOTA bifunctional chelator.

18F-FMISO or fluoromisonidazole is a radiopharmaceutical used for PET imaging of hypoxia. It consists of a 2-nitroimidazole molecule labelled with the positron-emitter fluorine-18.

Peptide receptor radionuclide therapy (PRRT) is a type of radionuclide therapy, using a radiopharmaceutical that targets peptide receptors to deliver localised treatment, typically for neuroendocrine tumours (NETs).

Somatostatin receptor antagonists are a class of chemical compounds that work by imitating the structure of the neuropeptide somatostatin, which is an endogenous hormone found in the human body. The somatostatin receptors are G protein-coupled receptors. Somatostatin receptor subtypes in humans include sstr1, 2A, 2 B, 3, 4, and 5. While normally expressed in the gastrointestinal (GI) tract, pancreas, hypothalamus, and central nervous system (CNS), they are expressed in different types of tumours. The predominant subtype in cancer cells is the ssrt2 subtype, which is expressed in neuroblastomas, meningiomas, medulloblastomas, breast carcinomas, lymphomas, renal cell carcinomas, paragangliomas, small cell lung carcinomas, and hepatocellular carcinomas.

CERN-MEDical Isotopes Collected from ISOLDE (MEDICIS) is a facility located in the Isotope Separator Online DEvice (ISOLDE) facility at CERN, designed to produce high-purity isotopes for developing the practice of patient diagnosis and treatment. The facility was initiated in 2010, with its first radioisotopes (terbium-155) produced on 12 December 2017.













