Coronary artery disease (CAD), also called coronary heart disease (CHD), ischemic heart disease (IHD), myocardial ischemia, or simply heart disease, involves the reduction of blood flow to the cardiac muscle due to build-up of atherosclerotic plaque in the arteries of the heart. It is the most common of the cardiovascular diseases. Types include stable angina, unstable angina, and myocardial infarction.

Angina, also known as angina pectoris, is chest pain or pressure, usually caused by insufficient blood flow to the heart muscle (myocardium). It is most commonly a symptom of coronary artery disease.

Chest pain is pain or discomfort in the chest, typically the front of the chest. It may be described as sharp, dull, pressure, heaviness or squeezing. Associated symptoms may include pain in the shoulder, arm, upper abdomen, or jaw, along with nausea, sweating, or shortness of breath. It can be divided into heart-related and non-heart-related pain. Pain due to insufficient blood flow to the heart is also called angina pectoris. Those with diabetes or the elderly may have less clear symptoms.
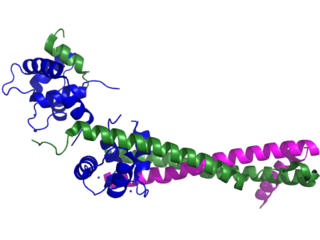
Troponin, or the troponin complex, is a complex of three regulatory proteins that are integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Measurements of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome. Blood troponin levels may be used as a diagnostic marker for stroke or other myocardial injury that is ongoing, although the sensitivity of this measurement is low.

A cardiac stress test is a cardiological examination that evaluates the cardiovascular system's response to external stress within a controlled clinical setting. This stress response can be induced through physical exercise or intravenous pharmacological stimulation of heart rate.

Cardiac markers are biomarkers measured to evaluate heart function. They can be useful in the early prediction or diagnosis of disease. Although they are often discussed in the context of myocardial infarction, other conditions can lead to an elevation in cardiac marker level.

Acute coronary syndrome (ACS) is a syndrome due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies. The most common symptom is centrally located pressure-like chest pain, often radiating to the left shoulder or angle of the jaw, and associated with nausea and sweating. Many people with acute coronary syndromes present with symptoms other than chest pain, particularly women, older people, and people with diabetes mellitus.

Unstable angina is a type of angina pectoris that is irregular or more easily provoked. It is classified as a type of acute coronary syndrome.

Acute pericarditis is a type of pericarditis usually lasting less than 6 weeks. It is the most common condition affecting the pericardium.

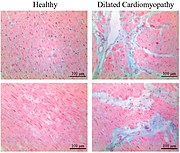
Takotsubo cardiomyopathy or takotsubo syndrome (TTS), also known as stress cardiomyopathy, is a type of non-ischemic cardiomyopathy in which there is a sudden temporary weakening of the muscular portion of the heart. It usually appears after a significant stressor, either physical or emotional; when caused by the latter, the condition is sometimes called broken heart syndrome.
Door-to-balloon is a time measurement in emergency cardiac care (ECC), specifically in the treatment of ST segment elevation myocardial infarction. The interval starts with the patient's arrival in the emergency department, and ends when a catheter guidewire crosses the culprit lesion in the cardiac cath lab. Because of the adage that "time is muscle", meaning that delays in treating a myocardial infarction increase the likelihood and amount of cardiac muscle damage due to localised hypoxia, ACC/AHA guidelines recommend a door-to-balloon interval of no more than 90 minutes. As of 2006 in the United States, fewer than half of STEMI patients received reperfusion with primary percutaneous coronary intervention (PCI) within the guideline-recommended timeframe. It has become a core quality measure for the Joint Commission on Accreditation of Healthcare Organizations (TJC).

Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to actin in relaxed muscle. When calcium binds to the troponin C, it causes conformational changes which lead to dislocation of troponin I. Afterwards, tropomyosin leaves the binding site for myosin on actin leading to contraction of muscle. The letter I is given due to its inhibitory character. It is a useful marker in the laboratory diagnosis of heart attack. It occurs in different plasma concentration but the same circumstances as troponin T - either test can be performed for confirmation of cardiac muscle damage and laboratories usually offer one test or the other.

Myocardial perfusion imaging or scanning is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium).

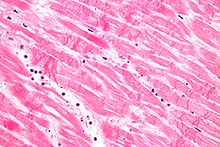
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops in one of the coronary arteries of the heart, causing infarction to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often such pain occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn.

Heart-type fatty acid binding protein (hFABP) also known as mammary-derived growth inhibitor is a protein that in humans is encoded by the FABP3 gene.

Electrocardiography in suspected myocardial infarction has the main purpose of detecting ischemia or acute coronary injury in emergency department populations coming for symptoms of myocardial infarction (MI). Also, it can distinguish clinically different types of myocardial infarction.

Francis Miller Fesmire was an American emergency physician and a nationally recognized expert in myocardial infarction. He authored numerous academic articles and assisted in the development of clinical guidelines on the standard of care in treating patients with suspected myocardial infarction by the American College of Emergency Physicians and the American Heart Association/American College of Cardiology. He performed numerous research investigations in chest pain patients, reporting the usefulness of continuous 12-lead ECG monitoring, two-hour delta cardiac marker testing, and nuclear cardiac stress testing in the emergency department. The culmination of his studies was The Erlanger Chest Pain Evaluation Protocol published in the Annals of Emergency Medicine in 2002. In 2011 he published a novel Nashville Skyline that received a 5 star review by ForeWord Reviews. His most recent research involved the risk stratification of chest pain patients in the emergency department.

Management of acute coronary syndrome is targeted against the effects of reduced blood flow to the affected area of the heart muscle, usually because of a blood clot in one of the coronary arteries, the vessels that supply oxygenated blood to the myocardium. This is achieved with urgent hospitalization and medical therapy, including drugs that relieve chest pain and reduce the size of the infarct, and drugs that inhibit clot formation; for a subset of patients invasive measures are also employed. Basic principles of management are the same for all types of acute coronary syndrome. However, some important aspects of treatment depend on the presence or absence of elevation of the ST segment on the electrocardiogram, which classifies cases upon presentation to either ST segment elevation myocardial infarction (STEMI) or non-ST elevation acute coronary syndrome (NST-ACS); the latter includes unstable angina and non-ST elevation myocardial infarction (NSTEMI). Treatment is generally more aggressive for STEMI patients, and reperfusion therapy is more often reserved for them. Long-term therapy is necessary for prevention of recurrent events and complications.
Kounis syndrome is defined as acute coronary syndrome caused by an allergic reaction or a strong immune reaction to a drug or other substance. It is a rare syndrome with authentic cases reported in 130 males and 45 females, as reviewed in 2017; however, the disorder is suspected of being commonly overlooked and therefore much more prevalent. Mast cell activation and release of inflammatory cytokines as well as other inflammatory agents from the reaction leads to spasm of the arteries leading to the heart muscle or a plaque breaking free and blocking one or more of those arteries.
Remote ischemic conditioning (RIC) is an experimental medical procedure that aims to reduce the severity of ischaemic injury to an organ such as the heart or the brain, most commonly in the situation of a heart attack or a stroke, or during procedures such as heart surgery when the heart may temporary suffer ischaemia during the operation, by triggering the body's natural protection against tissue injury. Although noted to have some benefits in experimental models in animals, this is still an experimental procedure in humans and initial evidence from small studies have not been replicated in larger clinical trials. Successive clinical trials have failed to identify evidence supporting a protective role in humans.