
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula [RhCl(PPh3)3] (Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use.

Vaska's complex is the trivial name for the chemical compound trans-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic organometallic complex consists of a central iridium atom bound to two mutually trans triphenylphosphine ligands, carbon monoxide and a chloride ion. The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability to bind to O2 reversibly. It is a bright yellow crystalline solid.

Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
The transition metal ruthenium forms several compounds, with oxidation states of ruthenium ranging from 0 to +8, and −2. The properties of ruthenium and osmium compounds are often similar. The +2, +3, and +4 states are the most common. The most prevalent precursor is ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically.
Martin Arthur Bennett FRS is an Australian inorganic chemist. He gained recognition for studies on the co-ordination chemistry of tertiary phosphines, olefins, and acetylenes, and the relationship of their behaviour to homogeneous catalysis.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

Organoiridium chemistry is the chemistry of organometallic compounds containing an iridium-carbon chemical bond. Organoiridium compounds are relevant to many important processes including olefin hydrogenation and the industrial synthesis of acetic acid. They are also of great academic interest because of the diversity of the reactions and their relevance to the synthesis of fine chemicals.
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early transition metals such as titanium, niobium, and tungsten. Oxophilicity is often stated to be related to the hardness of the element, within the HSAB theory, but it has been shown that oxophilicity depends more on the electronegativity and effective nuclear charge of the element than on its hardness. This explains why the early transition metals, whose electronegativities and effective nuclear charges are low, are very oxophilic. Many main group compounds are also oxophilic, such as derivatives of aluminium, silicon, and phosphorus(III). The handling of oxophilic compounds often requires air-free techniques.
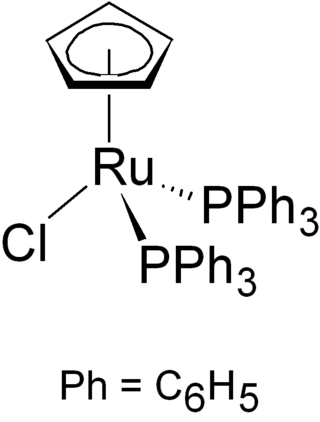
Chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium is the organoruthenium half-sandwich compound with formula RuCl(PPh3)2(C5H5). It as an air-stable orange crystalline solid that is used in a variety of organometallic synthetic and catalytic transformations. The compound has idealized Cs symmetry. It is soluble in chloroform, dichloromethane, and acetone.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.

A metal-phosphine complex is a In coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Carbonyl hydrido tris(triphenylphosphine)rhodium(I) [Carbonyl(hydrido)tris(triphenylphosphane)rhodium(I)] is an organorhodium compound with the formula [RhH(CO)(PPh3)3] (Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation.
In organic chemistry, the Murai reaction is an organic reaction that uses C-H activation to create a new C-C bond between a terminal or strained internal alkene and an aromatic compound using a ruthenium catalyst. The reaction, named after Shinji Murai, was first reported in 1993. While not the first example of C-H activation, the Murai reaction is notable for its high efficiency and scope. Previous examples of such hydroarylations required more forcing conditions and narrow scope.

Bis(triphenylphosphine)rhodium carbonyl chloride is the organorhodium complex with the formula [RhCl(CO)(PPh3)2]. This complex of rhodium(I) is a bright yellow, air-stable solid. It is the Rh analogue of Vaska's complex, the corresponding iridium complex. With regards to its structure, the complex is square planar with mutually trans triphenylphosphine (PPh3) ligands. The complex is a versatile homogeneous catalyst.
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand. The -PH2 ion or ligand is also called phosphanide or phosphido ligand.

Transition metal carbonate and bicarbonate complexes are coordination compounds containing carbonate (CO32-) and bicarbonate (HCO3-) as ligands. The inventory of complexes is large, enhanced by the fact that the carbonate ligand can bind metal ions in a variety of bonding modes.













