
Polypyridine complexes are coordination complexes containing polypyridine ligands, such as 2,2'-bipyridine, 1,10-phenanthroline, or 2,2';6'2"-terpyridine.

Bipyridines are a family of organic compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. Bipyridines, especially the 4,4' isomer, are mainly of significance in pesticides.

BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1′ positions. This C2-symmetric framework lacks a stereogenic atom, but has axial chirality due to restricted rotation (atropisomerism). The barrier to racemization is high due to steric hindrance, which limits rotation about the bond linking the naphthyl rings. The dihedral angle between the naphthyl groups is approximately 90°. The natural bite angle is 93°.

Photosensitizers are light absorbers that alter the course of a photochemical reaction. They usually are catalysts. They can function by many mechanisms, sometimes they donate an electron to the substrate, sometimes they abstract a hydrogen atom from the substrate. At the end of this process, the photosensitizer returns to its ground state, where it remains chemically intact, poised to absorb more light. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.
Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ultraviolet lamps are employed. Organic photochemistry has proven to be a very useful synthetic tool. Complex organic products can be obtained simply.
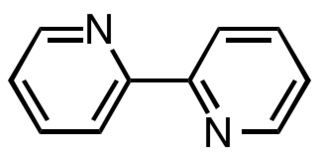
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications.
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.

Electrochemiluminescence or electrogenerated chemiluminescence (ECL) is a kind of luminescence produced during electrochemical reactions in solutions. In electrogenerated chemiluminescence, electrochemically generated intermediates undergo a highly exergonic reaction to produce an electronically excited state that then emits light upon relaxation to a lower-level state. This wavelength of the emitted photon of light corresponds to the energy gap between these two states. ECL excitation can be caused by energetic electron transfer (redox) reactions of electrogenerated species. Such luminescence excitation is a form of chemiluminescence where one/all reactants are produced electrochemically on the electrodes.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Stefan Bernhard is recognized in the scientific community for his work in several applied fields pertaining to the interaction between light and transition metal complexes. His involvement in the prediction, generation, and spectroscopy of circularly polarized luminescence from synthesized chiral phosphors have significantly advanced the state-of-the-art in this relatively young sub-field of photophysical chemistry. Other contributions involve work in artificial photosynthesis and organic light emitting devices.
Photochemical reduction of carbon dioxide harnesses solar energy to convert CO2 into higher-energy products. Environmental interest in producing artificial systems is motivated by recognition that CO2 is a greenhouse gas. The process has not been commercialized.

Tris(acetonitrile)cyclopentadienylruthenium hexafluorophosphate is an organoruthenium compound with the formula [(C5H5)Ru(NCCH3)3]PF6, abbreviated [CpRu(NCMe)3]PF6. It is a yellow-brown solid that is soluble in polar organic solvents. The compound is a salt consisting of the hexafluorophosphate anion and the cation [CpRu(NCMe)3]+. In coordination chemistry, it is used as a source of RuCp+ for further derivitization. In organic synthesis, it is a homogeneous catalyst. It enables C-C bond formation and promotes cycloadditions. The cyclopentadienyl ligand (Cp) is bonded in an η5 manner to the Ru(II) center.
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
Tehshik Peter Yoon is a Canadian-born chemist who studies the new reaction methods for organic synthesis with the use of catalysis. Yoon currently is a professor at the University of Wisconsin–Madison in the chemistry department. For his contributions to science, he has received numerous awards including the Beckman Young Investigator Award and National Science Foundation CAREER Award.
Photo-Induced Cross-Linking of Unmodified Proteins (PICUP) is a protein cross-linking method by visible light irradiation of a photocatalyst in the presence of an electron acceptor and the protein of interest. Irradiation results in a highly reactive protein radical that forms a covalent bond between the amino acid side chains of the proteins to be linked. Cross-linking methods developed prior to PICUP, including the use of physical, oxidative, and chemical cross-linkers, often require more time and result in protein byproducts. In addition, the cross-linked protein yield is very low due to the multifunctionality of the cross-linking reagents.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.
The Stahl oxidation is a copper-catalyzed aerobic oxidation of primary and secondary alcohols to aldehydes and ketones. Known for its high selectivity and mild reaction conditions, the Stahl oxidation offers several advantages over classical alcohol oxidations.
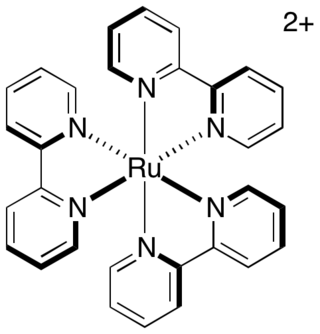
Transition metal complexes of 2,2'-bipyridine are coordination complexes containing one or more 2,2'-bipyridine ligands. Complexes have been described for all of the transition metals. Although few have any practical value, these complexes have been influential. 2,2'-Bipyridine is classified as a diimine ligand. Unlike the structures of pyridine complexes, the two rings in bipy are coplanar, which facilitates electron delocalization. As a consequence of this delocalization, bipy complexes often exhibit distinctive optical and redox properties.

Dichlororuthenium tricarbonyl dimer is an organoruthenium compound with the formula [RuCl2(CO)3]2. A yellow solid, the molecule features a pair of octahedral Ru centers bridged by a pair of chloride ligands. The complex is a common starting material in ruthenium chemistry.

Tris(bipyridine)iron(II) chloride is the chloride salt of the coordination complex tris(bipyridine)iron(II), [Fe(C10H8N2)3]2+. It is a red solid. In contrast to tris(bipyridine)ruthenium(II), this iron complex is not a useful photosensitizer because its excited states relax too rapidly, a consequence of the primogenic effect.



![Transitions of [Ru(bpy)3] Ruthenium bipyridyl energy level diagram.png](http://upload.wikimedia.org/wikipedia/commons/thumb/f/f1/Ruthenium_bipyridyl_energy_level_diagram.png/200px-Ruthenium_bipyridyl_energy_level_diagram.png)
![Absorption and emission spectrum of [Ru(bpy)3] in alcoholic solution at room temperature Ru(bpy)32+ absorption&emission.png](http://upload.wikimedia.org/wikipedia/commons/thumb/a/ae/Ru%28bpy%2932%2B_absorption%26emission.png/200px-Ru%28bpy%2932%2B_absorption%26emission.png)












