
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requiring a continuous source of fuel and oxygen to sustain the chemical reaction, whereas in a battery the chemical energy usually comes from substances that are already present in the battery. Fuel cells can produce electricity continuously for as long as fuel and oxygen are supplied.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
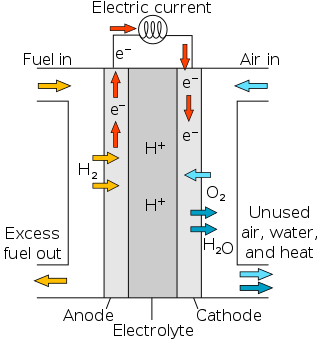
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.

An ionic liquid (IL) is a salt in the liquid state at ambient conditions. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.
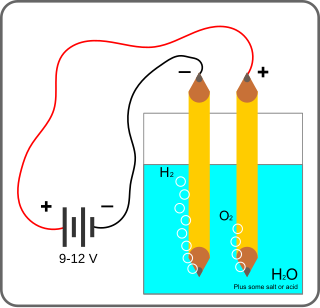
Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Formic acid fuel cells (direct formic acid fuel cells or DFAFCs) are a subcategory of direct liquid-feed fuel cells (DLFCs), in which the liquid fuel is directly oxidized (electrochemically) at the anode instead of reforming to produce hydrogen. Formic acid-based fuel cells represent a promising energy supply system in terms of high volumetric energy density, theoretical energy efficiency, and theoretical open-circuit voltage. They are also able to overcome certain problems inherent to traditional hydrogen (H2) feed fuel cells such as safe handling, storage, and H2 transportation.
Hydrogen purification is any technology used to purify hydrogen. The impurities in hydrogen gas depend on the source of the H2, e.g., petroleum, coal, electrolysis, etc. The required purity is determined by the applicatoin of the hydrogne gas. For example, ultra-high purified hydrogen is needed for applications like proton exchange membrane fuel cells.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
The electrochemical reduction of carbon dioxide, also known as CO2RR, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It represents one potential step in the broad scheme of carbon capture and utilization.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
Photoelectrochemical reduction of carbon dioxide, also known as photoelectrolysis of carbon dioxide, is a chemical process whereby carbon dioxide is reduced to carbon monoxide or hydrocarbons by the energy of incident light. This process requires catalysts, most of which are semiconducting materials. The feasibility of this chemical reaction was first theorised by Giacomo Luigi Ciamician, an Italian photochemist. Already in 1912 he stated that "[b]y using suitable catalyzers, it should be possible to transform the mixture of water and carbon dioxide into oxygen and methane, or to cause other endo-energetic processes."
Water oxidation is one of the half reactions of water splitting:

An alkaline anion-exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide-exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion-exchange membrane to separate the anode and cathode compartments.
A solar fuel is a synthetic chemical fuel produced from solar energy. Solar fuels can be produced through photochemical, photobiological, and electrochemical reactions.
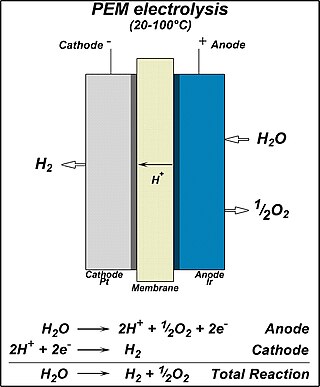
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
Karen Chan is an associate professor at the Technical University of Denmark. She is a Canadian and French physicist most notable for her work on catalysis, electrocatalysis, and electrochemical reduction of carbon dioxide.
The Bionic Leaf is a biomimetic system that gathers solar energy via photovoltaic cells that can be stored or used in a number of different functions. Bionic leaves can be composed of both synthetic and organic materials (bacteria), or solely made of synthetic materials. The Bionic Leaf has the potential to be implemented in communities, such as urbanized areas to provide clean air as well as providing needed clean energy.
Hydrogen evolution reaction (HER) is a chemical reaction that yields H2. The conversion of protons to H2 requires reducing equivalents and usually a catalyst. In nature, HER is catalyzed by hydrogenase enzymes. Commercial electrolyzers typically employ platinum supported as the catalyst at the anode of the electrolyzer. HER is useful for producing hydrogen gas, providing a clean-burning fuel. HER, however, can also be an unwelcome side reaction that competes with other reductions such as nitrogen fixation, or electrochemical reduction of carbon dioxide or chrome plating.

Anion exchange membrane(AEM) electrolysis is the electrolysis of water that utilises a semipermeable membrane that conducts hydroxide ions (OH−) called an anion exchange membrane. Like a proton-exchange membrane (PEM), the membrane separates the products, provides electrical insulation between electrodes, and conducts ions. Unlike PEM, AEM conducts hydroxide ions. The major advantage of AEM water electrolysis is that a high-cost noble metal catalyst is not required, low-cost transition metal catalyst can be used instead. AEM electrolysis is similar to alkaline water electrolysis, which uses a non-ion-selective separator instead of an anion-exchange membrane.











