
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current in a circuit is opposite to the direction of electron flow, so electrons flow from the anode of a galvanic cell, into an outside or external circuit connected to the cell. For example, the end of a household battery marked with a "+" is the cathode.
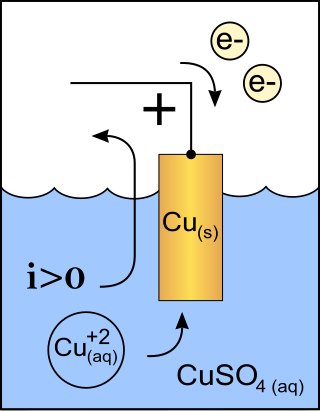
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode.

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
In electrochemistry, electrode potential is the voltage of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (SHE). It is defined to have a potential of zero volts. It may also be defined as the potential difference between the charged metallic rods and salt solution.
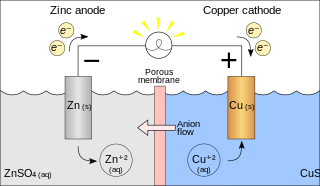
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.

An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would otherwise not occur. The external energy source is a voltage applied between the cell's two electrodes; an anode and a cathode, which are immersed in an electrolyte solution. This is in contrast to a galvanic cell, which itself is a source of electrical energy and the foundation of a battery. The net reaction taking place in a galvanic cell is a spontaneous reaction, i.e., the Gibbs free energy remains -ve, while the net reaction taking place in an electrolytic cell is the reverse of this spontaneous reaction, i.e., the Gibbs free energy is +ve.
In electrochemistry, standard electrode potential, or , is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as; "the value of the standard emf of a cell in which molecular hydrogen under standard pressure is oxidized to solvated protons at the left-hand electrode".

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
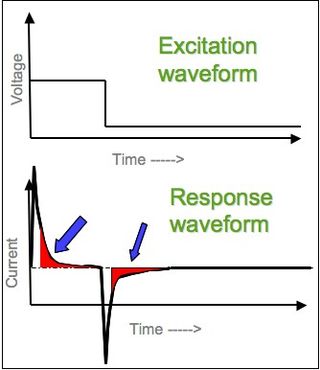
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
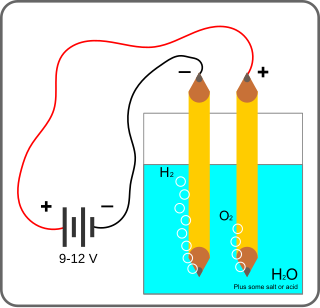
Électrolysis of
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.
In electrochemistry, Faraday efficiency describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reaction. The word "Faraday" in this term has two interrelated aspects: first, the historic unit for charge is the faraday (F), but has since been replaced by the coulomb (C); and secondly, the related Faraday's constant correlates charge with moles of matter and electrons. This phenomenon was originally understood through Michael Faraday's work and expressed in his laws of electrolysis.
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employs a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential (volts) and current (amps) is monitored over time (seconds). In a properly run experiment an analyte is quantitatively converted from its original oxidation state to a new oxidation state, either reduced or oxidized. As the substrate is consumed, the current also decreases, approaching zero when the conversion nears completion.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
In electrochemistry, exchange current density is a parameter used in the Tafel equation, Butler–Volmer equation and other electrochemical kinetics expressions. The Tafel equation describes the dependence of current for an electrolytic process to overpotential.
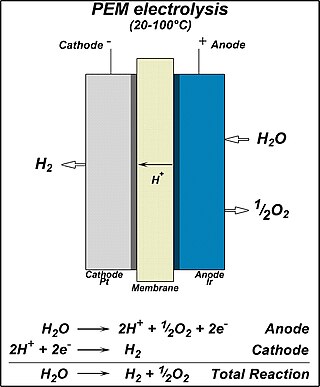
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
The Virtual breakdown mechanism is a concept in the field of electrochemistry. In electrochemical reactions, when the cathode and the anode are close enough to each other, the double layer of the regions from the two electrodes is overlapped, forming a large electric field uniformly distributed inside the entire electrode gap. Such high electric fields can significantly enhance the ion migration inside bulk solutions and thus increase the entire reaction rate, akin to the "breakdown" of the reactant(s). However, it is fundamentally different from the traditional "breakdown".












