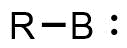In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele.

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.
Cycloheptene is a 7-membered cycloalkene with a flash point of −6.7 °C. It is a raw material in organic chemistry and a monomer in polymer synthesis. Cycloheptene can exist as either the cis- or the trans-isomer.
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.
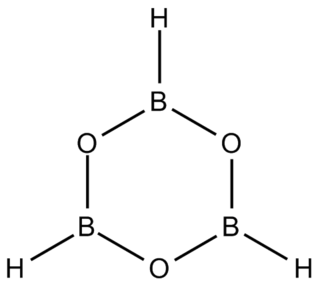
Boroxine is a 6-membered heterocyclic compound composed of alternating oxygen and singly-hydrogenated boron atoms. Boroxine derivatives such as trimethylboroxine and triphenylboroxine also make up a broader class of compounds called boroxines. These compounds are solids that are usually in equilibrium with their respective boronic acids at room temperature. Beside being used in theoretical studies, boroxine is primarily used in the production of optics.

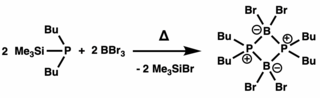
Phosphinidenes are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The "free" form of these compounds is conventionally described as having a singly-coordinated phosphorus atom containing only 6 electrons in its valence level. Most phosphinidenes are highly reactive and short-lived, thereby complicating empirical studies on their chemical properties. In the last few decades, several strategies have been employed to stabilize phosphinidenes, and researchers have developed a number of reagents and systems that can generate and transfer phosphinidenes as reactive intermediates in the synthesis of various organophosphorus compounds.
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom respectively. They are isoelectronic with the cyclopentadienyl cation C5H+5(Cp+) and comprise four π electrons. Although Hückel's rule cannot be strictly applied to borole, it is considered to be antiaromatic due to having 4 π electrons. As a result, boroles exhibit unique electronic properties not found in other metalloles.
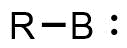
A borylene is the boron analogue of a carbene. The general structure is R-B: with R an organic moiety and B a boron atom with two unshared electrons. Borylenes are of academic interest in organoboron chemistry. A singlet ground state is predominant with boron having two vacant sp2 orbitals and one doubly occupied one. With just one additional substituent the boron is more electron deficient than the carbon atom in a carbene. For this reason stable borylenes are more uncommon than stable carbenes. Some borylenes such as boron monofluoride (BF) and boron monohydride (BH) the parent compound also known simply as borylene, have been detected in microwave spectroscopy and may exist in stars. Other borylenes exist as reactive intermediates and can only be inferred by chemical trapping.
Digermynes are a class of compounds that are regarded as the heavier digermanium analogues of alkynes. The parent member of this entire class is HGeGeH, which has only been characterized computationally, but has revealed key features of the whole class. Because of the large interatomic repulsion between two Ge atoms, only kinetically stabilized digermyne molecules can be synthesized and characterized by utilizing bulky protecting groups and appropriate synthetic methods, for example, reductive coupling of germanium(II) halides.
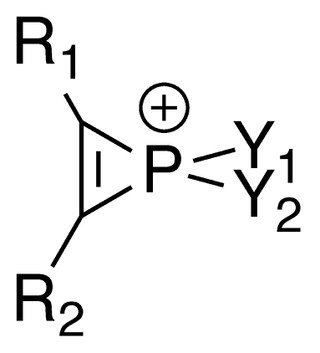
Phosphirenium ions are a series of organophosphorus compounds containing unsaturated three-membered ring phosphorus (V) heterocycles and σ*-aromaticity is believed to be present in such molecules. Many of the salts containing phosphirenium ions have been isolated and characterized by NMR spectroscopy and X-ray crystallography.

Diphosphagermylenes are a class of compounds containing a divalent germanium atom bound to two phosphorus atoms. While these compounds resemble diamidocarbenes, such as N-heterocyclic carbenes (NHC), diphosphagermylenes display bonding characteristics distinct from those of diamidocarbenes. In contrast to NHC compounds, in which there is effective N-C p(π)-p(π) overlap between the lone pairs of planar nitrogens and an empty p-orbital of a carbene, systems containing P-Ge p(π)-p(π) overlap are rare. Until 2014, the geometry of phosphorus atoms in all previously reported diphosphatetrylenes are pyramidal, with minimal P-Ge p(π)-p(π) interaction. It has been suggested that the lack of p(π)-p(π) in Ge-P bonds is due to the high energetic barrier associated with achieving a planar configuration at phosphorus, which would allow for efficient p(π)-p(π) overlap between the phosphorus lone pair and the empty P orbital of Ge. The resulting lack of π stabilization contributes to the difficulty associated with isolating diphosphagermylene and the Ge-P double bonds. However, utilization of sterically encumbering phosphorus centers has allowed for the isolation of diphosphagermylenes with a planar phosphorus center with a significant P-Ge p(π)-p(π) interaction.
A phosphetane is a 4-membered organophosphorus heterocycle. The parent phosphetane molecule, which has the formula C3H7P, is one atom larger than phosphiranes, one smaller than phospholes, and is the heavy-atom analogue of azetidines. The first known phosphetane synthesis was reported in 1957 by Kosolapoff and Struck, but the method was both inefficient and hard to reproduce, with yields rarely exceeding 1%. A far more efficient method was reported in 1962 by McBride, whose method allowed for the first studies into the physical and chemical properties of phosphetanes. Phosphetanes are a well understood class of molecules that have found broad applications as chemical building blocks, reagents for organic/inorganic synthesis, and ligands in coordination chemistry.

Silylones are a class of zero-valent monatomic silicon complexes, characterized as having two lone pairs and two donor-acceptor ligand interactions stabilizing a silicon(0) center. Synthesis of silylones generally involves the use of sterically bulky carbenes to stabilize highly reactive Si(0) centers. For this reason, silylones are sometimes referred to siladicarbenes. To date, silylones have been synthesized with cyclic alkyl amino carbenes (cAAC) and bidentate N-heterocyclic carbenes (bis-NHC). They are capable of reactions with a variety of substrates, including chalcogens and carbon dioxide.
Boron porphyrins are a variety of porphyrin, a common macrocycle used for photosensitization and metal trapping applications, that incorporate boron. The central four nitrogen atoms in a porphyrin macrocycle form a unique molecular pocket which is known to accommodate transition metals of various sizes and oxidation states. Due to the diversity of binding modes available to porphyrin, there is a growing interest in introducing other elements into this pocket.

Phosphenium ions, not to be confused with phosphonium or phosphirenium, are divalent cations of phosphorus of the form [PR2]+. Phosphenium ions have long been proposed as reaction intermediates.

The triboracyclopropenyl fragment is a cyclic structural motif in boron chemistry, named for its geometric similarity to cyclopropene. In contrast to nonplanar borane clusters that exhibit higher coordination numbers at boron (e.g., through 3-center 2-electron bonds to bridging hydrides or cations), triboracyclopropenyl-type structures are rings of three boron atoms where substituents at each boron are also coplanar to the ring. Triboracyclopropenyl-containing compounds are extreme cases of inorganic aromaticity. They are the lightest and smallest cyclic structures known to display the bonding and magnetic properties that originate from fully delocalized electrons in orbitals of σ and π symmetry. Although three-membered rings of boron are frequently so highly strained as to be experimentally inaccessible, academic interest in their distinctive aromaticity and possible role as intermediates of borane pyrolysis motivated extensive computational studies by theoretical chemists. Beginning in the late 1980s with mass spectrometry work by Anderson et al. on all-boron clusters, experimental studies of triboracyclopropenyls were for decades exclusively limited to gas-phase investigations of the simplest rings (ions of B3). However, more recent work has stabilized the triboracyclopropenyl moiety via coordination to donor ligands or transition metals, dramatically expanding the scope of its chemistry.

Nontrigonal pnictogen compounds refer to tricoordinate trivalent pnictogen compounds that are not of typical trigonal pyramidal molecular geometry. By virtue of their geometric constraint, these compounds exhibit distinct electronic structures and reactivities, which bestow on them potential to provide unique nonmetal platforms for bond cleavage reactions.
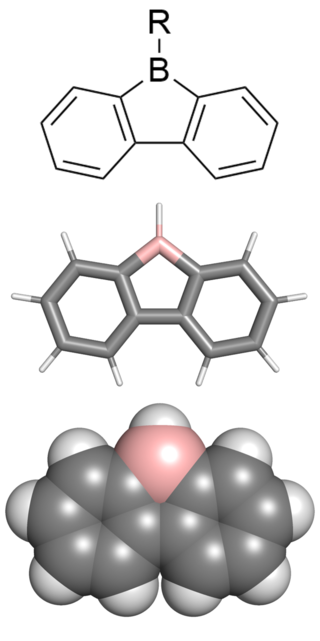
9-borafluorenes are a class of boron-containing heterocycles consisting of a tricyclic system with a central BC4 ring with two fused arene groups. 9-borafluorenes can be thought of as a borole with two fused arene rings, or as a trigonal planar boron atom with an empty p orbital bridging two biphenyl rings. However, 9-borafluorenes are generally less reactive than boroles due to less antiaromatic character and Lewis acidity. Containing highly conjugated π systems, 9-borafluorenes possess interesting photophysical properties. In addition, 9-borafluorenes are good Lewis acids. This combination of properties enables potential uses such as in light-emitting materials, solar cells, and sensors for some molecules.

An N-heterocyclic carbene boryl anion is an isoelectronic structure of an N-heterocyclic carbene (NHC), where the carbene carbon is replaced with a boron atom that has a -1 charge. NHC boryl anions have a planar geometry, and the boron atom is considered to be sp2-hybridized. They serve as extremely strong bases, as they are very nucleophilic. They also have a very strong trans influence, due to the σ-donation coming from the boron atom. NHC boryl anions have stronger electron-releasing character when compared to normal NHCs. These characteristics make NHC boryl anions key ligands in many applications, such as polycyclic aromatic hydrocarbons, and more commonly low oxidation state main group element bonding.
Heteroatomic multiple bonding between group 13 and group 15 elements are of great interest in synthetic chemistry due to their isoelectronicity with C-C multiple bonds. Nevertheless, the difference of electronegativity between group 13 and 15 leads to different character of bondings comparing to C-C multiple bonds. Because of the ineffective overlap between p𝝅 orbitals and the inherent lewis acidity/basicity of group 13/15 elements, the synthesis of compounds containing such multiple bonds is challenging and subject to oligomerization. The most common example of compounds with 13/15 group multiple bonds are those with B=N units. The boron-nitrogen-hydride compounds are candidates for hydrogen storage. In contrast, multiple bonding between aluminium and nitrogen Al=N, Gallium and nitrogen (Ga=N), boron and phosphorus (B=P), or boron and arsenic (B=As) are less common.