
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934%. It is more than twice as abundant as water vapor, 23 times as abundant as carbon dioxide, and more than 500 times as abundant as neon. Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.
Cyanogen is the chemical compound with the formula (CN)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒C≡N, although other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also Cyano radical.)
In chemistry, noble gas compounds are chemical compounds that include an element from the noble gases, group 18 of the periodic table. Although the noble gases are generally unreactive elements, many such compounds have been observed, particularly involving the element xenon.

Boron trioxide or diboron trioxide is the oxide of boron with the formula B2O3. It is a colorless transparent solid, almost always glassy (amorphous), which can be crystallized only with great difficulty. It is also called boric oxide or boria. It has many important industrial applications, chiefly in ceramics as a flux for glazes and enamels and in the production of glasses.
Diphosphene is a type of organophosphorus compound that has a phosphorus–phosphorus double bond, denoted by R-P=P-R'. These compounds are not common but are of theoretical interest. Normally, compounds with the empirical formula RP exist as rings. However, like other multiple bonds between heavy main-group elements, P=P double bonds can be stabilized by a large steric hindrance from the substitutions. The first isolated diphosphene bis(2,4,6-tri-tert-butylphenyl)diphosphene was exemplified by Masaaki Yoshifuji and his coworkers in 1981, in which diphosphene is stabilized by two bulky phenyl group.

Xenon hexafluoride is a noble gas compound with the formula XeF6. It is one of the three binary fluorides of xenon, the other two being XeF2 and XeF4. All known are exergonic and stable at normal temperatures. XeF6 is the strongest fluorinating agent of the series. It is a colorless solid that readily sublimes into intensely yellow vapors.

Glycolaldehyde is the organic compound with the formula HOCH2−CHO. It is the smallest possible molecule that contains both an aldehyde group and a hydroxyl group. It is a highly reactive molecule that occurs both in the biosphere and in the interstellar medium. It is normally supplied as a white solid. Although it conforms to the general formula for carbohydrates, Cn(H2O)n, it is not generally considered to be a saccharide.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.

Technetium hexafluoride or technetium(VI) fluoride (TcF6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. The other such compound is technetium(VI) chloride, TcCl6. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium (spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing.

Lithium amide or lithium azanide is an inorganic compound with the chemical formula LiNH2. It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
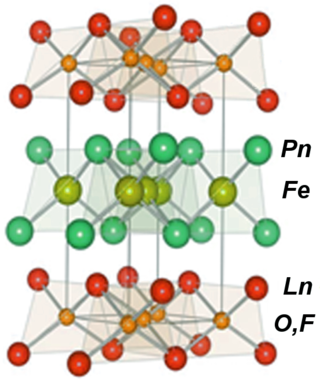
Iron-based superconductors (FeSC) are iron-containing chemical compounds whose superconducting properties were discovered in 2006. In 2008, led by recently discovered iron pnictide compounds, they were in the first stages of experimentation and implementation..

Magnesium hydride is the chemical compound with the molecular formula MgH2. It contains 7.66% by weight of hydrogen and has been studied as a potential hydrogen storage medium.

Neptunium(IV) oxide, or neptunium dioxide, is a radioactive, olive green cubic crystalline solid with the formula NpO2. It emits both α- and γ-particles.
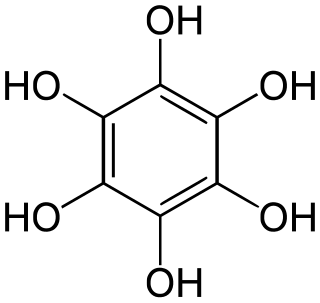
Benzenehexol, also called hexahydroxybenzene, is an organic compound with formula C6H6O6 or C6(OH)6. It is a six-fold phenol of benzene. The product is also called hexaphenol, but this name has been used also for other substances.
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.

Iron(I) hydride, systematically named iron hydride and poly(hydridoiron) is a solid inorganic compound with the chemical formula (FeH)
n. It is both thermodynamically and kinetically unstable toward decomposition at ambient temperature, and as such, little is known about its bulk properties.
Helium is the smallest and the lightest noble gas and one of the most unreactive elements, so it was commonly considered that helium compounds cannot exist at all, or at least under normal conditions. Helium's first ionization energy of 24.57 eV is the highest of any element. Helium has a complete shell of electrons, and in this form the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. The electron affinity is 0.080 eV, which is very close to zero. The helium atom is small with the radius of the outer electron shell at 0.29 Å. Helium is a very hard atom with a Pearson hardness of 12.3 eV. It has the lowest polarizability of any kind of atom, however, very weak van der Waals forces exist between helium and other atoms. This force may exceed repulsive forces, so at extremely low temperatures helium may form van der Waals molecules. Helium has the lowest boiling point of any known substance.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
Caesium sesquioxide is a chemical compound with the formula Cs2O3 or Cs4O6. In terms of oxidation states, Caesium in this compound has a nominal charge of +1, and the oxygen is a mixed peroxide and superoxide for a structural formula of (Cs+)4(O−2)2(O2−2). Compared to the other caesium oxides, this phase is less well studied, but has been long present in the literature. It can be created by thermal decomposition of caesium superoxide at 290 °C.












