Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

Hydroxylamine is an inorganic compound with the formula NH2OH. The material is a white crystalline, hygroscopic compound. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. The oxidation of NH3 to hydroxylamine is a step in biological nitrification.

Nitrous acid is a weak and monoprotic acid known only in solution, in the gas phase and in the form of nitrite salts. It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.

Sodium azide is an inorganic compound with the formula NaN3. This colorless salt is the gas-forming component in some car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance, is highly soluble in water, and is very acutely poisonous.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

Chloroplatinic acid (also known as hexachloroplatinic acid) is an inorganic compound with the formula [H3O]2[PtCl6](H2O)x (0 ≤ x ≤ 6). A red solid, it is an important commercial source of platinum, usually as an aqueous solution. Although often written in shorthand as H2PtCl6, it is the hydronium (H3O+) salt of the hexachloroplatinate anion (PtCl2−
6). Hexachloroplatinic acid is highly hygroscopic.

Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
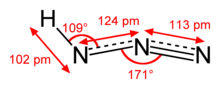
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.

Imidazole-1-sulfonyl azide is an organic azide compound that can be used as an alternative organic synthesis reagent to trifluoromethanesulfonyl azide. It is an explosive colorless liquid, but some of its organic-soluble salts can be safely handled and stored as a solid.

In chemistry, the pentazenium cation is a positively-charged polyatomic ion with the chemical formula N+5 and structure N−N−N−N−N. Together with solid nitrogen polymers and the azide anion, it is one of only three poly-nitrogen species obtained in bulk quantities.
Barium azide is an inorganic azide with the formula Ba(N3)2. It is a barium salt of hydrazoic acid. Like most azides, it is explosive. It is less sensitive to mechanical shock than lead azide.
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen atoms covalently bonded to hydrogen.

Rubidium azide is an inorganic compound with the formula RbN3. It is the rubidium salt of the hydrazoic acid HN3. Like most azides, it is explosive.
Hydrazinium azide or hydrazine azide is a chemical compound with formula H
5N
5 or [N
2H+
5][N−
3]. It is a salt of the hydrazinium cation N
2H+
5 and the azide anion N−
3. It can be seen as a derivative of hydrazine N
2H
4 and hydrazoic acid HN
3. It is an unstable solid.
Zinc azideZn(N3)2 is an inorganic compound composed of zinc cations (Zn2+) and azide anions (N−3). It is a white, explosive solid that can be prepared by the protonolysis of diethylzinc with hydrazoic acid:

Boron triazide, also known as triazidoborane, is a thermally unstable compound of boron and nitrogen with a nitrogen content of 92.1 %. Formally, it is the triazido derivative of borane and is a covalent inorganic azide. The high-energy compound, which has the propensity to undergo spontaneous explosive decomposition, was first described in 1954 by Egon Wiberg and Horst Michaud of the University of Munich.
Homoleptic azido compounds are chemical compounds in which the only anion or ligand is the azide group, -N3. The breadth of homoleptic azide compounds spans nearly the entire periodic table. With rare exceptions azido compounds are highly shock sensitive and need to be handled with the upmost caution. Binary azide compounds can take on several different structures including discrete compounds, or one- two, and three-dimensional nets, leading some to dub them as "polyazides". Reactivity studies of azide compounds are relatively limited due to how sensitive they can be. The sensitivity of these compounds tends to be correlated with the amount of ionic or covalent character the azide-element bond has, with ionic character being far more stable than covalent character. Therefore, compounds such as silver or sodium azide – which have strong ionic character – tend to possess more synthetic utility than their covalent counterparts. A few other notable exceptions include polymeric networks which possess unique magnetic properties, group 13 azides which unlike most other azides decompose to nitride compounds (important materials for semiconductors), other limited uses as synthetic reagents for the transfer for azide groups, or interest in high energy density materials.