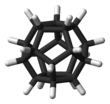
Cyclopentadiene is an organic compound with the formula C5H6. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2023. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.
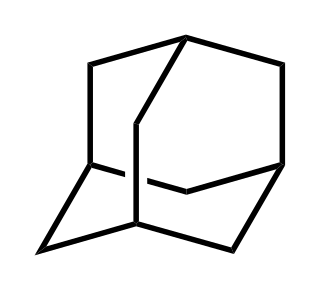
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon–carbon bonds and the Greek word tropos, meaning turn. In this type of rearrangement reaction, a substituent moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the [3,3] Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis.
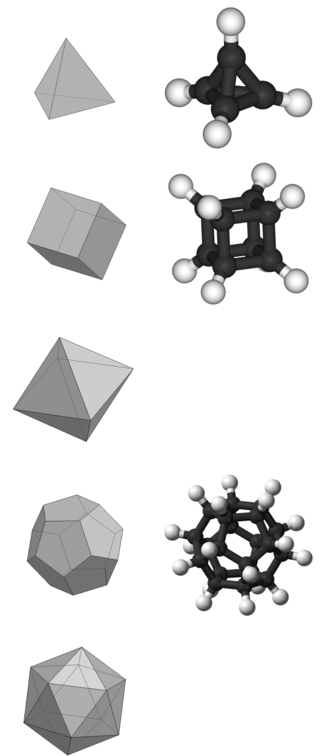
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed.

Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in the name reflects the notion of a small molecule trapped inside a shell. Two types of endohedral complexes exist: endohedral metallofullerenes and non-metal doped fullerenes.

Sumanene is a polycyclic aromatic hydrocarbon and of scientific interest because the molecule can be considered a fragment of buckminsterfullerene. Suman means "sunflower" in both Hindi and Sanskrit. The core of the arene is a benzene ring and the periphery consists of alternating benzene rings (3) and cyclopentadiene rings (3). Unlike fullerene, sumanene has benzyl positions which are available for organic reactions.
Endohedral hydrogen fullerene (H2@C60) is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis methods.
A Norrish reaction, named after Ronald George Wreyford Norrish, is a photochemical reaction taking place with ketones and aldehydes. Such reactions are subdivided into Norrish type I reactions and Norrish type II reactions. While of limited synthetic utility these reactions are important in the photo-oxidation of polymers such as polyolefins, polyesters, certain polycarbonates and polyketones.

Carbon tetraiodide is a tetrahalomethane with the molecular formula CI4. Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2.3% by weight carbon, although other methane derivatives are known with still less carbon.

Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example, fullerene is notoriously insoluble and adding a suitable group can enhance solubility. By adding a polymerizable group, a fullerene polymer can be obtained. Functionalized fullerenes are divided into two classes: exohedral fullerenes with substituents outside the cage and endohedral fullerenes with trapped molecules inside the cage.

The Kuwajima Taxol total synthesis by the group of Isao Kuwajima of the Tokyo Institute of Technology is one of several efforts in taxol total synthesis published in the 1990s. The total synthesis of Taxol is considered a landmark in organic synthesis.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.

A dyotropic reaction in organic chemistry is a type of organic reaction and more specifically a pericyclic valence isomerization in which two sigma bonds simultaneously migrate intramolecularly. The reaction type is of some relevance to organic chemistry because it can explain how certain reactions occur and because it is a synthetic tool in the synthesis of organic molecules for example in total synthesis. It was first described by Manfred T. Reetz in 1971 In a type I reaction two migrating groups interchange their relative positions and a type II reaction involves migration to new bonding sites without positional interchange.

Pagodane is an organic compound with formula C
20H
20 whose carbon skeleton was said to resemble a pagoda, hence the name. It is a polycyclic hydrocarbon whose molecule has the D2h point symmetry group. The compound is a highly crystalline solid that melts at 243 °C, is barely soluble in most organic solvents and moderately soluble in benzene and chloroform. It sublimes at low pressure.

Olympicene is an organic carbon-based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings.
Horst Prinzbach was a German chemist and professor emeritus.
Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene).