Ecdysone is a prohormone of the major insect molting hormone 20-hydroxyecdysone, secreted from the prothoracic glands. It is of steroidal structure. Insect molting hormones are generally called ecdysteroids. Ecdysteroids act as moulting hormones of arthropods but also occur in other related phyla where they can play different roles. In Drosophila melanogaster, an increase in ecdysone concentration induces the expression of genes coding for proteins that the larva requires. It causes chromosome puffs to form in polytene chromosomes. Recent findings in the laboratory of Chris Q. Doe have found a novel role of this hormone in regulating temporal gene transitions within neural stem cells of the fruit fly.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.
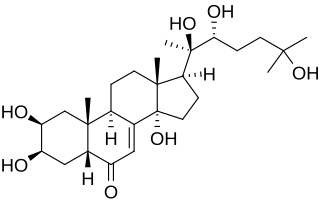
20-Hydroxyecdysone is a naturally occurring ecdysteroid hormone which controls the ecdysis (moulting) and metamorphosis of arthropods. It is therefore one of the most common moulting hormones in insects, crabs, etc. It is also a phytoecdysteroid produced by various plants, including Cyanotis vaga, Ajuga turkestanica and Rhaponticum carthamoides where its purpose is presumably to disrupt the development and reproduction of insect pests. In arthropods, 20-hydroxyecdysone acts through the ecdysone receptor. Although mammals lack this receptor, 20-hydroxyecdysone affects mammalian biological systems. 20-Hydroxyecdysone is an ingredient of some supplements that aim to enhance physical performance. In humans, it is hypothesized to bind to the estrogen receptor beta (ERβ) protein-coding gene.

Oxandrolone is an androgen and synthetic anabolic steroid (AAS) medication to help promote weight gain in various situations, to help offset protein catabolism caused by long-term corticosteroid therapy, to support recovery from severe burns, to treat bone pain associated with osteoporosis, to aid in the development of girls with Turner syndrome, and for other indications. It is taken by mouth. It was sold under the brand names Oxandrin and Anavar, among others.
Glycogen synthase is a key enzyme in glycogenesis, the conversion of glucose into glycogen. It is a glycosyltransferase that catalyses the reaction of UDP-glucose and n to yield UDP and n+1.

Metandienone, also known as methandienone or methandrostenolone and sold under the brand name Dianabol (D-Bol) among others, is an androgen and anabolic steroid (AAS) medication which is still quite often used because of its affordability and effectiveness for bulking cycles. It is also used non-medically for physique- and performance-enhancing purposes. It is often taken by mouth.
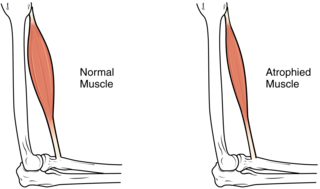
Muscle atrophy is the loss of skeletal muscle mass. It can be caused by immobility, aging, malnutrition, medications, or a wide range of injuries or diseases that impact the musculoskeletal or nervous system. Muscle atrophy leads to muscle weakness and causes disability.

Trenbolone acetate, sold under brand names such as Finajet and Finaplix among others, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, specifically to increase the profitability of livestock by promoting muscle growth in cattle. It is given by injection into muscle.

Muscle hypertrophy or muscle building involves a hypertrophy or increase in size of skeletal muscle through a growth in size of its component cells. Two factors contribute to hypertrophy: sarcoplasmic hypertrophy, which focuses more on increased muscle glycogen storage; and myofibrillar hypertrophy, which focuses more on increased myofibril size. It is the primary focus of bodybuilding-related activities.
Phytoecdysteroids are plant-derived ecdysteroids. Phytoecdysteroids are a class of chemicals that plants synthesize for defense against phytophagous insects. These compounds are mimics of hormones used by arthropods in the molting process known as ecdysis. When insects eat the plants with these chemicals they may prematurely molt, lose weight, or suffer other metabolic damage and die.
Ecdysone 20-monooxygenase (EC 1.14.99.22) is an enzyme that catalyzes the chemical reaction

Ribosomal protein S6 kinase beta-1 (S6K1), also known as p70S6 kinase, is an enzyme that in humans is encoded by the RPS6KB1 gene. It is a serine/threonine kinase that acts downstream of PIP3 and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway. As the name suggests, its target substrate is the S6 ribosomal protein. Phosphorylation of S6 induces protein synthesis at the ribosome.
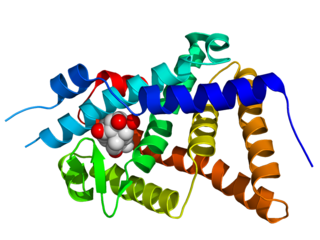
The ecdysone receptor is a nuclear receptor found in arthropods, where it controls development and contributes to other processes such as reproduction. The receptor is a non-covalent heterodimer of two proteins, the EcR protein and ultraspiracle protein (USP). It binds to and is activated by ecdysteroids. Insect ecdysone receptors are currently better characterized than those from other arthropods, and mimics of ecdysteroids are used commercially as caterpillar-selective insecticides.

Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor. Anabolic steroids have a number of medical uses, but are also used by athletes to increase muscle size, strength, and performance.

The halloween genes are a set of genes identified in Drosophila melanogaster that influence embryonic development. All of the genes code for cytochrome P450 enzymes in the ecdysteroidogenic pathway (biosynthesis of ecdysone from cholesterol). Ecdysteroids such as 20-hydroxyecdysone and ecdysone influence many of the morphological, physiological, biochemical changes that occur during molting in insects.
mTOR Complex 2 (mTORC2) is an acutely rapamycin-insensitive protein complex formed by serine/threonine kinase mTOR that regulates cell proliferation and survival, cell migration and cytoskeletal remodeling. The complex itself is rather large, consisting of seven protein subunits. The catalytic mTOR subunit, DEP domain containing mTOR-interacting protein (DEPTOR), mammalian lethal with sec-13 protein 8, and TTI1/TEL2 complex are shared by both mTORC2 and mTORC1. Rapamycin-insensitive companion of mTOR (RICTOR), mammalian stress-activated protein kinase interacting protein 1 (mSIN1), and protein observed with rictor 1 and 2 (Protor1/2) can only be found in mTORC2. Rictor has been shown to be the scaffold protein for substrate binding to mTORC2.
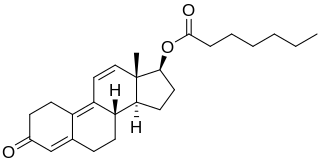
Trenbolone enanthate, known by the nickname Trenabol, is a synthetic and injected anabolic–androgenic steroid (AAS) and a derivative of nandrolone which was never marketed. It is the C17β enanthate ester and a long-acting prodrug of trenbolone. Trenbolone enanthate was never approved for medical or veterinary use but is used in scientific research and has been sold on the internet black market as a designer steroid for bodybuilders and athletes.
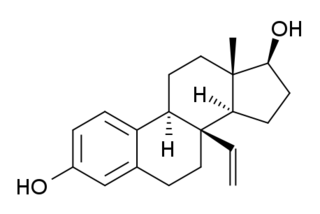
8β-VE2, or 8β-vinylestradiol, also known as 8β-vinylestra-1,3,5(10)-triene-3β,17β-diol, is a synthetic estrogen featuring an estradiol core. It is a highly potent and selective agonist of the ERβ that is used in scientific research to study the function of the ERβ. It has 190-fold higher potency in transactivation assays of the ERβ relative to the ERα and 93- (rat) and 180-fold (human) preference in binding affinity for the ERβ over the ERα.

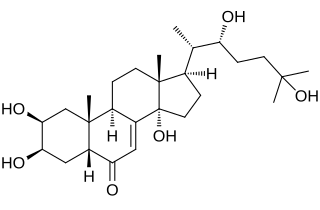
Turkesterone is a phytoecdysteroid found in numerous plant species, including Ajuga turkestanica, various Vitex species, Triticum aestivum, and Rhaponticum acaule.















