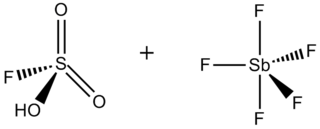In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative atom or group, and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn–H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F).

Methyl is an organic compound with the chemical formula CH•
3. It is a metastable colourless gas, which is mainly produced in situ as a precursor to other hydrocarbons in the petroleum cracking industry. It can act as either a strong oxidant or a strong reductant, and is quite corrosive to metals.

A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5 and vinyl C
2H+
3 cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered.
The bond-dissociation energy (BDE, D0, or DH°) is one measure of the strength of a chemical bond A–B. It can be defined as the standard enthalpy change when A–B is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane (C2H6) is defined as the standard enthalpy change of the process
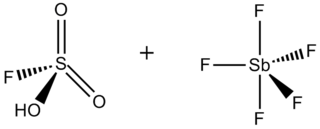
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted–Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.

Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride, containing various cations and anions. This substance is a superacid that can be excess of a million billion times stronger than 100% pure sulfuric acid, depending on proportion of its ingredients. It has been shown to protonate even hydrocarbons to afford pentacoordinate carbocations. Extreme caution needs to be in place when handling fluoroantimonic acid. It is exceptionally corrosive, but can be stored in containers lined with PTFE (Teflon).
In chemistry, a carbonium ion is any cation that has a pentavalent carbon atom. The name carbonium may also be used for the simplest member of the class, properly called methanium, where the five valences are filled with hydrogen atoms.
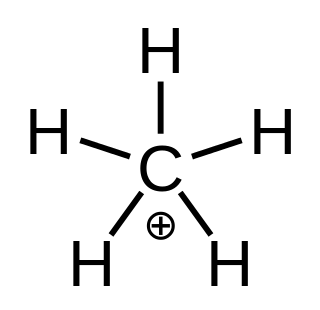
In chemistry, methanium is a complex positive ion with formula [CH
3(H
2)]+, namely a molecule with one carbon atom bonded to three hydrogen atoms and one hydrogen molecule, bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion.
Heat of formation group additivity methods in thermochemistry enable the calculation and prediction of heat of formation of organic compounds based on additivity. This method was pioneered by S. W. Benson.
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake for the class, is ammonium, NH+
4, the protonated derivative of ammonia, NH3.
Fluxional molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling. Where such movement does not occur, the molecule may be described as a semi-rigid molecule. Longuet-Higgins introduced the use of permutation-inversion groups for the symmetry classification of the states of fluxional molecules.

Atomic carbon, systematically named carbon and λ0-methane, also called monocarbon, is a colourless gaseous inorganic chemical with the chemical formula C. It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation.

The helium hydride ion or hydridohelium(1+) ion or helonium is a cation (positively charged ion) with chemical formula HeH+. It consists of a helium atom bonded to a hydrogen atom, with one electron removed. It can also be viewed as protonated helium. It is the lightest heteronuclear ion, and is believed to be the first compound formed in the Universe after the Big Bang.
Methylene is an organic compound with the chemical formula CH
2. It is a colourless gas that fluoresces in the mid-infrared range, and only persists in dilution, or as an adduct.

In organic chemistry, methenium is a cation with the formula CH+
3. It can be viewed as a methylene radical with an added proton, or as a methyl radical with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions.
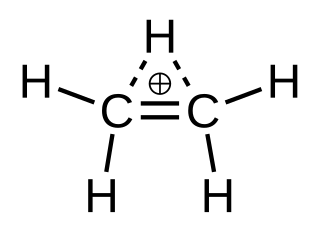
In chemistry, ethenium, protonated ethylene or ethyl cation is a positive ion with the formula C
2H+
5. It can be viewed as a molecule of ethylene with one added proton, or a molecule of ethane minus one hydride ion. It is a carbocation; more specifically, a nonclassical carbocation.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.

Argonium (also called the argon hydride cation, the hydridoargon(1+) ion, or protonated argon; chemical formula ArH+) is a cation combining a proton and an argon atom. It can be made in an electric discharge, and was the first noble gas molecular ion to be found in interstellar space.
In chemistry, the decay technique is a method to generate chemical species such as radicals, carbocations, and other potentially unstable covalent structures by radioactive decay of other compounds. For example, decay of a tritium-labeled molecule yields an ionized helium atom, which might then break off to leave a cationic molecular fragment.