Aromatic compounds are those chemical compounds that contain one or more rings with pi electrons delocalized all the way around them. In contrast to compounds that exhibit aromaticity, aliphatic compounds lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. Aromatic hydrocarbons, or arenes, are aromatic organic compounds containing solely carbon and hydrogen atoms. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simple aromatic compound benzene, or a phenyl group when part of a larger compound.

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Hydrocarbons are generally colourless and hydrophobic with only weak odours. Because of their diverse molecular structures, it is difficult to generalize further. Most anthropogenic emissions of hydrocarbons are from the burning of fossil fuels including fuel production and combustion. Natural sources of hydrocarbons such as ethylene, isoprene, and monoterpenes come from the emissions of vegetation.

In chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with pi bonds in resonance that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability.
A polycyclic aromatic hydrocarbon (PAH) is a hydrocarbon—a chemical compound containing only carbon and hydrogen—that is composed of multiple aromatic rings. The group is a major subset of the aromatic hydrocarbons. The simplest of such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. The terms polyaromatic hydrocarbon or polynuclear aromatic hydrocarbon are also used for this concept.

In chemistry, the organic compound triphenylene is a flat polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings. Triphenylene can be isolated from coal tar. It is also made synthetically by synthesis and trimerization of benzyne. One molecule of triphenylene has delocalized 18-π-electron systems based on a planar structure. It has the molecular formula C
18H
12.

Idrialite is a rare hydrocarbon mineral with approximate chemical formula C22H14.
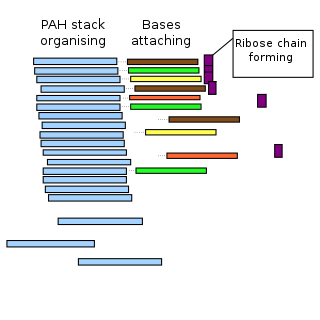
The PAH world hypothesis is a speculative hypothesis that proposes that polycyclic aromatic hydrocarbons (PAHs), known to be abundant in the universe, including in comets, and assumed to be abundant in the primordial soup of the early Earth, played a major role in the origin of life by mediating the synthesis of RNA molecules, leading into the RNA world. However, as yet, the hypothesis is untested.

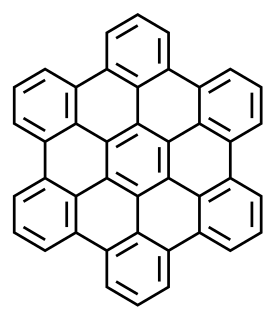
Carpathite is a very rare hydrocarbon mineral, consisting of exceptionally pure coronene (C24H12), a polycyclic aromatic hydrocarbon. The name has been spelled karpatite and the mineral was improperly renamed pendletonite.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.

Chrysene is a polycyclic aromatic hydrocarbon (PAH) with the molecular formula C
18H
12 that consists of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote at levels of 0.5-6 mg/kg.
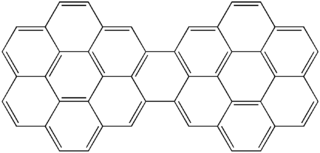
Dicoronylene is the trivial name for a very large polycyclic aromatic hydrocarbon. Its formal name is benzo[10,11]phenanthro[2',3',4',5',6':4,5,6,7]chryseno[1,2,3-bc]coronene or benzo[1,2,3-bc:4,5,6-b'c']dicoronene. It has 15 rings and is a brick-red solid. Its formula is C
48H
20. Dicoronylene sublimes under high vacuum, 0.001 torr, between 250 °C and 300 °C.

Superheated water is liquid water under pressure at temperatures between the usual boiling point, 100 °C (212 °F) and the critical temperature, 374 °C (705 °F). It is also known as "subcritical water" or "pressurized hot water." Superheated water is stable because of overpressure that raises the boiling point, or by heating it in a sealed vessel with a headspace, where the liquid water is in equilibrium with vapour at the saturated vapor pressure. This is distinct from the use of the term superheating to refer to water at atmospheric pressure above its normal boiling point, which has not boiled due to a lack of nucleation sites.
Chlorinated polycyclic aromatic hydrocarbons (Cl-PAHs) are a group of compounds comprising polycyclic aromatic hydrocarbons with two or more aromatic rings and one or more chlorine atoms attached to the ring system. Cl-PAHs can be divided into two groups: chloro-substituted PAHs, which have one or more hydrogen atoms substituted by a chlorine atom, and chloro-added Cl-PAHs, which have two or more chlorine atoms added to the molecule. They are products of incomplete combustion of organic materials. They have many congeners, and the occurrences and toxicities of the congeners differ. Cl-PAHs are hydrophobic compounds and their persistence within ecosystems is due to their low water solubility. They are structurally similar to other halogenated hydrocarbons such as polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and polychlorinated biphenyls (PCBs). Cl-PAHs in the environment are strongly susceptible to the effects of gas/particle partitioning, seasonal sources, and climatic conditions.

Benzo[j]fluoranthene (BjF) is an organic compound with the chemical formula C20H12. Classified as a polycyclic aromatic hydrocarbon (PAH), it is a colourless solid that is poorly soluble in most solvents. Impure samples can appear off white. Closely related isomeric compounds include benzo[a]fluoranthene (BaF), bendo[b]fluoranthene (BbF), benzo[e]fluoranthene (BeF), and benzo[k]fluoranthene (BkF). BjF is present in fossil fuels and is released during incomplete combustion of organic matter. It has been traced in the smoke of cigarettes, exhaust from gasoline engines, emissions from the combustion of various types of coal and emissions from oil heating, as well as an impurity in some oils such as soybean oil.
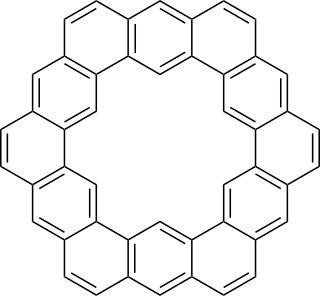
Kekulene is a polycyclic aromatic hydrocarbon and a circulene with the chemical formula C48H24. It was first synthesized in 1978, and was named in honor of August Kekulé, the discoverer of the structure of the benzene molecule.
The unidentified infrared emission bands are infrared discrete emissions from circumstellar regions, interstellar media, star-forming regions and extragalactic objects for which the identity of the emitting materials is unknown. The main infrared features occur around 3.3, 6.2, 7.7, 8.6, 11.2, and 12.7 μm, although there are many other weak emission features within the ~ 5–18 μm spectral range. In the 1980s, astronomers discovered that the origin of the UIR emission bands is inherent in compounds made of aromatic C–H and C=C chemical bonds, and hypothesized that the materials responsible should be polycyclic aromatic hydrocarbon (PAH) molecules. Nevertheless, data recorded with the ESA's Infrared Space Observatory and NASA's Spitzer Space Telescope have suggested that the UIR emission bands arise from compounds that are far more complex in composition and structure than PAH molecules. Moreover, the UIR bands follow a clear evolutionary spectral trend that is linked to the lifespan of the astronomical source; from the time the UIR bands first appear around evolved stars in the protoplanetary nebula stage to evolved stages such as the planetary nebula phase.
Vibrio cyclitrophicus is a polycyclic aromatic hydrocarbon (PAH)-degrading marine bacterium. The type strain is P-2P44T.
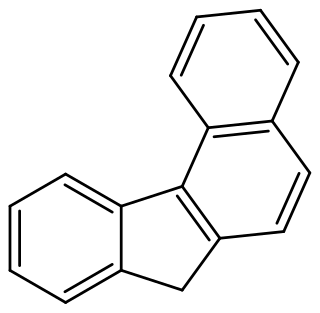
Benzo[c]fluorene is a polycyclic aromatic hydrocarbon (PAH) with mutagenic activity. It is a component of coal tar, cigarette smoke and smog and thought to be a major contributor to its carcinogenic properties. The mutagenicity of benzo[c]fluorene is mainly attributed to formation of metabolites that are reactive and capable of forming DNA adducts. According to the KEGG it is a group 3 carcinogen. Other names for benzo[c]fluorene are 7H-benzo[c]fluorene, 3,4-benzofluorene, and NSC 89264.

Contorted aromatics or more precisely contorted polycyclic aromatic hydrocarbons are polycyclic aromatic hydrocarbons (PAHs) in which the fused aromatic molecules deviate from the usual planarity.

Isoarborinol is a triterpenoid ubiquitously produced by angiosperms and is thus considered a biomarker for higher plants. Though no isoarborinol-producing microbe has been identified, isoarborinol is also considered a possible biomarker for marine bacteria, as its diagenetic end product, arborane, has been found in ancient marine sediments that predate the rise of plants. Importantly, isoarborinol may represent the phylogenetic link between hopanols and sterols.

















