Related Research Articles

The endocrine system is a messenger system comprising feedback loops of the hormones released by internal glands of an organism directly into the circulatory system, regulating distant target organs. In vertebrates, the hypothalamus is the neural control center for all endocrine systems. In humans, the major endocrine glands are the thyroid gland, parathyroid gland, pituitary gland, pineal gland, the testes (male), ovaries (female), and the adrenal glands. The hypothalamus, pancreas, and thymus also function as endocrine glands, among other functions. Other organs, such as the kidneys, also have roles within the endocrine system by secreting certain hormones. The study of the endocrine system and its disorders is known as endocrinology.
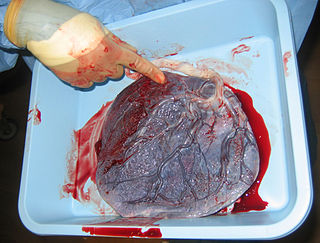
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth. Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development.

The hypothalamic–pituitary–adrenal axis is a complex set of direct influences and feedback interactions among three components: the hypothalamus, the pituitary gland, and the adrenal glands. These organs and their interactions constitute the HPA axis.

Pregnancy is the time during which one or more offspring develops (gestates) inside a woman's uterus (womb). A multiple pregnancy involves more than one offspring, such as with twins.
Environmental toxicants and fetal development is the impact of different toxic substances from the environment on the development of the fetus. This article deals with potential adverse effects of environmental toxicants on the prenatal development of both the embryo or fetus, as well as pregnancy complications. The human embryo or fetus is relatively susceptible to impact from adverse conditions within the mother's environment. Substandard fetal conditions often cause various degrees of developmental delays, both physical and mental, for the growing baby. Although some variables do occur as a result of genetic conditions pertaining to the father, a great many are directly brought about from environmental toxins that the mother is exposed to.
Prenatal development includes the development of the embryo and of the fetus during a viviparous animal's gestation. Prenatal development starts with fertilization, in the germinal stage of embryonic development, and continues in fetal development until birth.

Complications of pregnancy are health problems that are related to, or arise during pregnancy. Complications that occur primarily during childbirth are termed obstetric labor complications, and problems that occur primarily after childbirth are termed puerperal disorders. While some complications improve or are fully resolved after pregnancy, some may lead to lasting effects, morbidity, or in the most severe cases, maternal or fetal mortality.

Nutrition and pregnancy refers to the nutrient intake, and dietary planning that is undertaken before, during and after pregnancy. Nutrition of the fetus begins at conception. For this reason, the nutrition of the mother is important from before conception as well as throughout pregnancy and breastfeeding. An ever-increasing number of studies have shown that the nutrition of the mother will have an effect on the child, up to and including the risk for cancer, cardiovascular disease, hypertension and diabetes throughout life.
Metabolic imprinting refers to the long-term physiological and metabolic effects that an offspring's prenatal and postnatal environments have on them. Perinatal nutrition has been identified as a significant factor in determining an offspring's likelihood of it being predisposed to developing cardiovascular disease, obesity, and type 2 diabetes amongst other conditions.
Prenatal stress is exposure of an expectant mother to psychosocial or physical stress, which can be caused by daily life events or by environmental hardships. This psychosocial or physical stress that the expectant mother is experiencing has an effect on the fetus. According to the Developmental Origins of Health and Disease (DOHaD), a wide range of environmental factors a woman may experience during the perinatal period can contribute to biological impacts and changes in the fetus that then causes health risks later in the child's life.
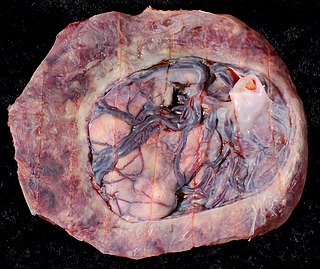
Circumvallate placenta is a rare condition affecting about 1-2% of pregnancies, in which the amnion and chorion fetal membranes essentially "double back" on the fetal side around the edges of the placenta. After delivery, a circumvallate placenta has a thick ring of membranes on its fetal surface. Circumvallate placenta is a placental morphological abnormality associated with increased fetal morbidity and mortality due to the restricted availability of nutrients and oxygen to the developing fetus.

Maternal physiological changes in pregnancy are the adaptations that take place during pregnancy that enable the accommodation of the developing embryo and fetus. These are normal physiological adaptations that cause changes in behavior, the functioning of the heart, blood vessels, and blood, metabolism including increases in blood sugar levels, kidney function, posture, and breathing. During pregnancy numerous hormones and proteins are secreted that also have a broad range of effects.

Prenatal nutrition addresses nutrient recommendations before and during pregnancy. Nutrition and weight management before and during pregnancy has a profound effect on the development of infants. This is a rather critical time for healthy development since infants rely heavily on maternal stores and nutrient for optimal growth and health outcome later in life.
Prenatal memory, also called fetal memory, is important for the development of memory in humans. Many factors can impair fetal memory and its functions, primarily maternal actions. There are multiple techniques available not only to demonstrate the existence of fetal memory but to measure it. Fetal memory is vulnerable to certain diseases so much so that exposure can permanently damage the development of the fetus and even terminate the pregnancy by aborting the fetus. Maternal nutrition and the avoidance of drugs, alcohol and other substances during all nine months of pregnancy is important to the development of the fetus and its memory systems. The use of certain substances can entail long-term permanent effects on the fetus that can carry on throughout their lifespan.

A high-risk pregnancy is one where the mother or the fetus has an increased risk of adverse outcomes compared to uncomplicated pregnancies. No concrete guidelines currently exist for distinguishing “high-risk” pregnancies from “low-risk” pregnancies; however, there are certain studied conditions that have been shown to put the mother or fetus at a higher risk of poor outcomes. These conditions can be classified into three main categories: health problems in the mother that occur before she becomes pregnant, health problems in the mother that occur during pregnancy, and certain health conditions with the fetus.
The fetal endocrine system is one of the first systems to develop during prenatal development of a human individual. The endocrine system arises from all three embryonic germ layers. The endocrine glands that produce the steroid hormones, such as the gonads and adrenal cortex, arise from the mesoderm. In contrast, endocrine glands that arise from the endoderm and ectoderm produce the amine, peptide, and protein hormones.
The fetal origins hypothesis proposes that the period of gestation has significant impacts on the developmental health and wellbeing outcomes for an individual ranging from infancy to adulthood. The effects of fetal origin are marked by three characteristics: latency, wherein effects may not be apparent until much later in life; persistency, whereby conditions resulting from a fetal effect continue to exist for a given individual; and genetic programming, which describes the 'switching on' of a specific gene due to prenatal environment. Research in the areas of economics, epidemiology, and epigenetics offer support for the hypothesis.

Medical imaging in pregnancy may be indicated because of pregnancy complications, intercurrent diseases or routine prenatal care.
Maternal fetal stress transfer is a physiological phenomenon in which psychosocial stress experienced by a mother during her pregnancy can be transferred to the fetus. Psychosocial stress describes the brain's physiological response to perceived social threat. Because of a link in blood supply between a mother and fetus, it has been found that stress can leave lasting effects on a developing fetus, even before a child is born. According to recent studies, these effects are mainly the result of two particular stress biomarkers circulating in the maternal blood supply: cortisol and catecholamines.
COVID-19 impact on pregnant women is the prenatal maternal stress that COVID-19 places the fetus and on the expectant mother. This impact can include psychosocial or physical stress caused by daily life events or by environmental hardships. Mental health issues, such as maternal depression, affect 10-20% of women and are linked to a variety of negative child outcomes. Prenatal stress has been demonstrated to affect the critical development stages in postnatal life that persist throughout adulthood. Health risks include impaired cognitive development, low birth weight, and risk of mental disorders in the offspring. Epigenetics may also be associated with the biological processes involved in prenatal stress, possibly leading to fetal programming.
References
- 1 2 Fleming TP, Velazquez MA, Eckert JJ, Lucas ES, Watkins AJ (February 2012). "Nutrition of females during the peri-conceptional period and effects on foetal programming and health of offspring". Animal Reproduction Science. 130 (3–4): 193–7. doi:10.1016/j.anireprosci.2012.01.015. PMID 22341375.
- ↑ Talge NM, Neal C, Glover V (March 2007). "Antenatal maternal stress and long-term effects on child neurodevelopment: how and why?". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 48 (3–4): 245–61. doi:10.1111/j.1469-7610.2006.01714.x. PMID 17355398.
- ↑ Remacle C, Bieswal F, Reusens B (November 2004). "Programming of obesity and cardiovascular disease". International Journal of Obesity and Related Metabolic Disorders. 28 Suppl 3 (S3): S46-53. doi: 10.1038/sj.ijo.0802800 . PMID 15543219.
- ↑ Taylor PD, Samuelsson AM, Poston L (March 2014). "Maternal obesity and the developmental programming of hypertension: a role for leptin". Acta Physiologica. 210 (3): 508–23. doi: 10.1111/apha.12223 . PMID 24433239. S2CID 22295003.
- ↑ Myatt L (April 2006). "Placental adaptive responses and fetal programming". The Journal of Physiology. 572 (Pt 1): 25–30. doi:10.1113/jphysiol.2006.104968. PMC 1779654 . PMID 16469781.
- 1 2 3 4 Hoffman MC (July 2016). "Stress, the Placenta, and Fetal Programming of Behavior: Genes' First Encounter With the Environment". The American Journal of Psychiatry. 173 (7): 655–7. doi: 10.1176/appi.ajp.2016.16050502 . PMID 27363547.
- 1 2 Andersen SL, Olsen J, Laurberg P (December 2015). "Foetal programming by maternal thyroid disease". Clinical Endocrinology. 83 (6): 751–8. doi: 10.1111/cen.12744 . PMID 25682985. S2CID 32873121.
- ↑ Moisiadis VG, Matthews SG (July 2014). "Glucocorticoids and fetal programming part 2: Mechanisms". Nature Reviews. Endocrinology. 10 (7): 403–11. doi:10.1038/nrendo.2014.74. PMID 24863383. S2CID 11475810.
- ↑ O'Donnell KJ, Meaney MJ (April 2017). "Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis". The American Journal of Psychiatry. 174 (4): 319–328. doi: 10.1176/appi.ajp.2016.16020138 . PMID 27838934.
- ↑ Kapoor A, Petropoulos S, Matthews SG (March 2008). "Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids". Brain Research Reviews. 57 (2): 586–95. doi:10.1016/j.brainresrev.2007.06.013. PMID 17716742. S2CID 30865698.
- ↑ Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA (May 2012). "Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems". Proceedings of the National Academy of Sciences of the United States of America. 109 (20): E1312-9. doi: 10.1073/pnas.1201295109 . PMC 3356611 . PMID 22529357.
- ↑ Graham AM, Rasmussen JM, Entringer S, Ben Ward E, Rudolph MD, Gilmore JH, et al. (January 2019). "Maternal Cortisol Concentrations During Pregnancy and Sex-Specific Associations With Neonatal Amygdala Connectivity and Emerging Internalizing Behaviors". Biological Psychiatry. 85 (2): 172–181. doi:10.1016/j.biopsych.2018.06.023. PMC 6632079 . PMID 30122286.
- ↑ Travers S, Martinerie L, Boileau P, Xue QY, Lombès M, Pussard E (April 2018). "Comparative profiling of adrenal steroids in maternal and umbilical cord blood". The Journal of Steroid Biochemistry and Molecular Biology. 178: 127–134. doi:10.1016/j.jsbmb.2017.11.012. PMID 29191401. S2CID 3705475.
- ↑ Chapman K, Holmes M, Seckl J (July 2013). "11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action". Physiological Reviews. 93 (3): 1139–206. doi:10.1152/physrev.00020.2012. PMC 3962546 . PMID 23899562.
- ↑ Stirrat LI, Sengers BG, Norman JE, Homer NZ, Andrew R, Lewis RM, Reynolds RM (February 2018). "Transfer and Metabolism of Cortisol by the Isolated Perfused Human Placenta". The Journal of Clinical Endocrinology and Metabolism. 103 (2): 640–648. doi:10.1210/jc.2017-02140. PMC 5800837 . PMID 29161409.
- 1 2 Ishimoto H, Jaffe RB (June 2011). "Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit". Endocrine Reviews. 32 (3): 317–55. doi:10.1210/er.2010-0001. PMC 3365797 . PMID 21051591.
- 1 2 3 4 5 6 Suter MA, Anders AM, Aagaard KM (January 2013). "Maternal smoking as a model for environmental epigenetic changes affecting birthweight and fetal programming". Molecular Human Reproduction. 19 (1): 1–6. doi:10.1093/molehr/gas050. PMC 3521486 . PMID 23139402.
- 1 2 3 Davis EP, Hankin BL, Swales DA, Hoffman MC (August 2018). "An experimental test of the fetal programming hypothesis: Can we reduce child ontogenetic vulnerability to psychopathology by decreasing maternal depression?". Development and Psychopathology. 30 (3): 787–806. doi:10.1017/S0954579418000470. PMC 7040571 . PMID 30068416.
- 1 2 Bekdash R, Zhang C, Sarkar D (September 2014). "Fetal alcohol programming of hypothalamic proopiomelanocortin system by epigenetic mechanisms and later life vulnerability to stress". Alcoholism: Clinical and Experimental Research. 38 (9): 2323–30. doi:10.1111/acer.12497. PMC 4177357 . PMID 25069392.
- 1 2 3 4 5 Weinberg J, Sliwowska JH, Lan N, Hellemans KG (April 2008). "Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome". Journal of Neuroendocrinology. 20 (4): 470–88. doi:10.1111/j.1365-2826.2008.01669.x. PMC 8942074 . PMID 18266938. S2CID 4574957.
- 1 2 3 Bayliss H, Churchill D, Beevers M, Beevers DG (January 2002). "Anti-hypertensive drugs in pregnancy and fetal growth: evidence for "pharmacological programming" in the first trimester?". Hypertension in Pregnancy. 21 (2): 161–74. doi:10.1081/prg-120013785. PMID 12175444. S2CID 30016072.