Related Research Articles
A microsatellite is a tract of repetitive DNA in which certain DNA motifs are repeated, typically 5–50 times. Microsatellites occur at thousands of locations within an organism's genome. They have a higher mutation rate than other areas of DNA leading to high genetic diversity. Microsatellites are often referred to as short tandem repeats (STRs) by forensic geneticists and in genetic genealogy, or as simple sequence repeats (SSRs) by plant geneticists.

Fragile X syndrome (FXS) is a genetic disorder characterized by mild-to-moderate intellectual disability. The average IQ in males with FXS is under 55, while about two thirds of affected females are intellectually disabled. Physical features may include a long and narrow face, large ears, flexible fingers, and large testicles. About a third of those affected have features of autism such as problems with social interactions and delayed speech. Hyperactivity is common, and seizures occur in about 10%. Males are usually more affected than females.
In genetics, anticipation is a phenomenon whereby as a genetic disorder is passed on to the next generation, the symptoms of the genetic disorder become apparent at an earlier age with each generation. In most cases, an increase in the severity of symptoms is also noted. Anticipation is common in trinucleotide repeat disorders, such as Huntington's disease and myotonic dystrophy, where a dynamic mutation in DNA occurs. All of these diseases have neurological symptoms. Prior to the understanding of the genetic mechanism for anticipation, it was debated whether anticipation was a true biological phenomenon or whether the earlier age of diagnosis was related to heightened awareness of disease symptoms within a family.

Non-Mendelian inheritance is any pattern in which traits do not segregate in accordance with Mendel's laws. These laws describe the inheritance of traits linked to single genes on chromosomes in the nucleus. In Mendelian inheritance, each parent contributes one of two possible alleles for a trait. If the genotypes of both parents in a genetic cross are known, Mendel's laws can be used to determine the distribution of phenotypes expected for the population of offspring. There are several situations in which the proportions of phenotypes observed in the progeny do not match the predicted values.

Haploinsufficiency in genetics describes a model of dominant gene action in diploid organisms, in which a single copy of the wild-type allele at a locus in heterozygous combination with a variant allele is insufficient to produce the wild-type phenotype. Haploinsufficiency may arise from a de novo or inherited loss-of-function mutation in the variant allele, such that it yields little or no gene product. Although the other, standard allele still produces the standard amount of product, the total product is insufficient to produce the standard phenotype. This heterozygous genotype may result in a non- or sub-standard, deleterious, and (or) disease phenotype. Haploinsufficiency is the standard explanation for dominant deleterious alleles.
Trinucleotide repeat disorders, a subset of microsatellite expansion diseases, are a set of over 30 genetic disorders caused by trinucleotide repeat expansion, a kind of mutation in which repeats of three nucleotides increase in copy numbers until they cross a threshold above which they cause developmental, neurological or neuromuscular disorders. Depending on its location, the unstable trinucleotide repeat may cause defects in a protein encoded by a gene; change the regulation of gene expression; produce a toxic RNA, or lead to production of a toxic protein. In general, the larger the expansion the faster the onset of disease, and the more severe the disease becomes.
The Sherman paradox was a term used to describe the anomalous pattern of inheritance found in fragile X syndrome. The phenomenon is also referred to as anticipation or dynamic mutation.

FMR1 is a human gene that codes for a protein called fragile X messenger ribonucleoprotein, or FMRP. This protein, most commonly found in the brain, is essential for normal cognitive development and female reproductive function. Mutations of this gene can lead to fragile X syndrome, intellectual disability, premature ovarian failure, autism, Parkinson's disease, developmental delays and other cognitive deficits. The FMR1 premutation is associated with a wide spectrum of clinical phenotypes that affect more than two million people worldwide.
Primary ovarian insufficiency (POI), also called premature ovarian insufficiency, premature menopause, and premature ovarian failure, is the partial or total loss of reproductive and hormonal function of the ovaries before age 40 because of follicular dysfunction or early loss of eggs. POI can be seen as part of a continuum of changes leading to menopause that differ from age-appropriate menopause in the age of onset, degree of symptoms, and sporadic return to normal ovarian function. POI affects approximately 1 in 10,000 women under age 20, 1 in 1,000 women under age 30, and 1 in 100 of those under age 40. A medical triad for the diagnosis is amenorrhea, hypergonadotropism, and hypoestrogenism.
A trinucleotide repeat expansion, also known as a triplet repeat expansion, is the DNA mutation responsible for causing any type of disorder categorized as a trinucleotide repeat disorder. These are labelled in dynamical genetics as dynamic mutations. Triplet expansion is caused by slippage during DNA replication, also known as "copy choice" DNA replication. Due to the repetitive nature of the DNA sequence in these regions, 'loop out' structures may form during DNA replication while maintaining complementary base pairing between the parent strand and daughter strand being synthesized. If the loop out structure is formed from the sequence on the daughter strand this will result in an increase in the number of repeats. However, if the loop out structure is formed on the parent strand, a decrease in the number of repeats occurs. It appears that expansion of these repeats is more common than reduction. Generally, the larger the expansion the more likely they are to cause disease or increase the severity of disease. Other proposed mechanisms for expansion and reduction involve the interaction of RNA and DNA molecules.

CGG triplet repeat-binding protein 1 is a protein that in humans is encoded by the CGGBP1 gene.
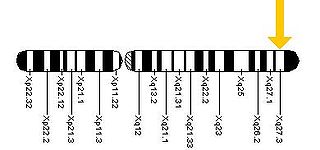
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a late-onset neurodegenerative disorder most frequently seen in male premutation carriers of Fragile X syndrome (FXS) over the age of 50. The main clinical features of FXTAS include problems of movement with cerebellar gait ataxia and action tremor. Associated features include parkinsonism, cognitive decline, and dysfunction of the autonomic nervous system. FXTAS is found in Fragile X "premutation" carriers, which is defined as a trinucleotide repeat expansion of 55-200 CGG repeats in the Fragile X mental retardation-1 (FMR1) gene. 4-40 CGG repeats in this gene is considered normal, while individual with >200 repeats have full Fragile X Syndrome.

In molecular biology, FMR1 antisense RNA 1 (FMR1-AS1), also known as ASFMR1 or FMR4, is a long non-coding RNA. The FMR1-AS1 gene overlaps, and is antisense to, the CGG repeat region of the FMR1 gene. Its expression is upregulated in fragile X syndrome premutation carriers, and silenced in patients with fragile X syndrome. FMR1-AS1 has an anti-apoptotic function.
A hereditary cancer syndrome is a genetic disorder in which inherited genetic mutations in one or more genes predispose the affected individuals to the development of cancer and may also cause early onset of these cancers. Hereditary cancer syndromes often show not only a high lifetime risk of developing cancer, but also the development of multiple independent primary tumors.
Nagwa Abdel Meguid is an Egyptian geneticist and 2002 winner of the L’Oreal UNESCO Award for Women in Science for Africa and the Middle East. Her research has "identified several genetic mutations that cause common syndromes such as the fragile X syndrome and Autism".
RNA-dominant diseases are characterized by deleterious mutations that typically result in degenerative disorders affecting various neurological, cardiovascular, and muscular functions. Studies have found that they arise from repetitive non-coding RNA sequences, also known as toxic RNA, which inhibit RNA-binding proteins leading to pathogenic effects. The most studied RNA-dominant diseases include, but are not limited to, myotonic dystrophy and fragile X-associated tremor/ataxia syndrome (FXTAS).
Randi J. Hagerman is an American physician who is the medical director of MIND Institute at the University of California, Davis. She works for the pediatrics department under the division of child development and behavior. She is an internationally recognized researcher in the field of genetics of autism spectrum disorder with special focus on genomic instability. Along with her husband Paul Hagerman, she discovered the Fragile X-associated tremor/ataxia syndrome (FXTAS), a neurological disorder that affects older male and rare female carriers of fragile X.
David L. Nelson is an American human geneticist, currently an associate director at the Intellectual and Developmental Disabilities Research Center (1995), and professor at the Department of Molecular and Human Genetics at Baylor College of Medicine BCM since 1999. Since 2018, he is the director at the Cancer and Cell Biology Ph.D program, and the director of Integrative Molecular and Biomedical Sciences Ph.D since 2015 at BCM.
Stephen T. Warren was an American geneticist and academic. He was the William Patterson Timmie Professor of Human Genetics and the Charles Howard Candler Chair of Human Genetics. He was the former Founding Chairman of the Department of Human Genetics at Emory University School of Medicine. He was an Investigator with the Howard Hughes Medical Institute from 1991 until 2002, when he resigned to found the Human Genetics department. Warren is well known for his work in the field of Human Genetics. His research was focused on the mechanistic understanding of fragile X syndrome, a leading cause of inherited developmental disability and autism. In 2020, Warren stepped down as department chair after 20 years in that position.

Oculopharyngodistal myopathy is a rare genetic disorder characterized by progressive muscle weakness affecting various parts of the body.
References
- ↑ Sullivan SD, Welt C, Sherman S (July 2011). "FMR1 and the continuum of primary ovarian insufficiency". Seminars in Reproductive Medicine. 29 (4): 299–307. doi:10.1055/s-0031-1280915. PMID 21969264.
- ↑ Allen EG, Charen K, Hipp HS, Shubeck L, Amin A, He W, et al. (September 2021). "Refining the risk for fragile X-associated primary ovarian insufficiency (FXPOI) by FMR1 CGG repeat size". Genetics in Medicine. 23 (9): 1648–1655. doi:10.1038/s41436-021-01177-y. PMC 8460441 . PMID 33927378.
- ↑ Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D (July 2012). "Prevalence of CGG expansions of the FMR1 gene in a US population-based sample". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 159B (5): 589–597. doi:10.1002/ajmg.b.32065. PMC 3391968 . PMID 22619118.
- 1 2 Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, et al. (August 2007). "Examination of reproductive aging milestones among women who carry the FMR1 premutation". Human Reproduction. 22 (8): 2142–2152. doi: 10.1093/humrep/dem148 . PMID 17588953.
- ↑ Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, et al. (October 2007). "Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines". Movement Disorders. 22 (14): 2018–30, quiz 2140. doi: 10.1002/mds.21493 . PMID 17618523. S2CID 12559110.
- ↑ Nolin SL, Brown WT, Glicksman A, Houck GE, Gargano AD, Sullivan A, et al. (February 2003). "Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles". American Journal of Human Genetics. 72 (2): 454–464. doi:10.1086/367713. PMC 379237 . PMID 12529854.
- 1 2 Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. (February 2005). "Association of FMR1 repeat size with ovarian dysfunction". Human Reproduction. 20 (2): 402–412. doi: 10.1093/humrep/deh635 . PMID 15608041.
- ↑ Nelson LM (February 2009). "Clinical practice. Primary ovarian insufficiency". The New England Journal of Medicine. 360 (6): 606–614. doi:10.1056/NEJMcp0808697. PMC 2762081 . PMID 19196677.
- 1 2 3 Hipp HS, Charen KH, Spencer JB, Allen EG, Sherman SL (September 2016). "Reproductive and gynecologic care of women with fragile X primary ovarian insufficiency (FXPOI)". Menopause. 23 (9): 993–999. doi:10.1097/GME.0000000000000658. PMC 4998843 . PMID 27552334.
- ↑ França MM, Mendonca BB (February 2020). "Genetics of Primary Ovarian Insufficiency in the Next-Generation Sequencing Era". Journal of the Endocrine Society. 4 (2): bvz037. doi: 10.1210/jendso/bvz037 . PMC 7033037 . PMID 32099950.
- ↑ Heddar A, Ogur C, Da Costa S, Braham I, Billaud-Rist L, Findlinki N, et al. (September 2022). "Genetic landscape of a large cohort of Primary Ovarian Insufficiency: New genes and pathways and implications for personalized medicine". eBioMedicine. 84: 104246. doi:10.1016/j.ebiom.2022.104246. PMC 9475279 . PMID 36099812.
- ↑ Mailick MR, Hong J, Greenberg J, Smith L, Sherman S (December 2014). "Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 165B (8): 705–711. doi:10.1002/ajmg.b.32277. PMC 4410868 . PMID 25346430.
- ↑ Allen EG, Glicksman A, Tortora N, Charen K, He W, Amin A, et al. (2018). "FXPOI: Pattern of AGG Interruptions Does not Show an Association With Age at Amenorrhea Among Women With a Premutation". Frontiers in Genetics. 9: 292. doi: 10.3389/fgene.2018.00292 . PMC 6086008 . PMID 30123240.
- 1 2 3 4 Yrigollen CM, Martorell L, Durbin-Johnson B, Naudo M, Genoves J, Murgia A, et al. (2014-07-30). "AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission". Journal of Neurodevelopmental Disorders. 6 (1): 24. doi: 10.1186/1866-1955-6-24 . PMC 4126815 . PMID 25110527.
- ↑ Villate O, Ibarluzea N, Maortua H, de la Hoz AB, Rodriguez-Revenga L, Izquierdo-Álvarez S, Tejada MI (2020). "Effect of AGG Interruptions on FMR1 Maternal Transmissions". Frontiers in Molecular Biosciences. 7: 135. doi: 10.3389/fmolb.2020.00135 . PMC 7381193 . PMID 32766278.
- 1 2 Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, et al. (January 2004). "Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population". JAMA. 291 (4): 460–469. doi:10.1001/jama.291.4.460. PMID 14747503.
- 1 2 Hagerman RJ, Protic D, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, Schneider A (2018-11-13). "Fragile X-Associated Neuropsychiatric Disorders (FXAND)". Frontiers in Psychiatry. 9: 564. doi: 10.3389/fpsyt.2018.00564 . PMC 6243096 . PMID 30483160.
- ↑ Wheeler AC, Bailey DB, Berry-Kravis E, Greenberg J, Losh M, Mailick M, et al. (December 2014). "Associated features in females with an FMR1 premutation". Journal of Neurodevelopmental Disorders. 6 (1): 30. doi: 10.1186/1866-1955-6-30 . PMC 4121434 . PMID 25097672.
- ↑ Mailick MR, Hong J, Movaghar A, DaWalt L, Berry-Kravis EM, Brilliant MH, et al. (October 2021). "Mild Neurological Signs in FMR1 Premutation Women in an Unselected Community-Based Cohort". Movement Disorders. 36 (10): 2378–2386. doi:10.1002/mds.28683. PMC 8597892 . PMID 34117786.
- ↑ Allen EG, Charen K, Hipp HS, Shubeck L, Amin A, He W, et al. (2021-10-01). "Predictors of Comorbid Conditions in Women Who Carry an FMR1 Premutation". Frontiers in Psychiatry. 12: 715922. doi: 10.3389/fpsyt.2021.715922 . PMC 8517131 . PMID 34658954.
- ↑ Gossett A, Sansone S, Schneider A, Johnston C, Hagerman R, Tassone F, et al. (December 2016). "Psychiatric disorders among women with the fragile X premutation without children affected by fragile X syndrome". American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 171 (8): 1139–1147. doi:10.1002/ajmg.b.32496. PMC 6907071 . PMID 27615674.
- ↑ Klusek J, Fairchild A, Moser C, Mailick MR, Thurman AJ, Abbeduto L (January 2022). "Family history of FXTAS is associated with age-related cognitive-linguistic decline among mothers with the FMR1 premutation". Journal of Neurodevelopmental Disorders. 14 (1): 7. doi: 10.1186/s11689-022-09415-3 . PMC 8903682 . PMID 35026985.
- 1 2 3 4 5 Nolin SL, Glicksman A, Tortora N, Allen E, Macpherson J, Mila M, et al. (July 2019). "Expansions and contractions of the FMR1 CGG repeat in 5,508 transmissions of normal, intermediate, and premutation alleles". American Journal of Medical Genetics. Part A. 179 (7): 1148–1156. doi:10.1002/ajmg.a.61165. PMC 6619443 . PMID 31050164.
- ↑ Willemsen R, Levenga J, Oostra BA (September 2011). "CGG repeat in the FMR1 gene: size matters". Clinical Genetics. 80 (3): 214–225. doi:10.1111/j.1399-0004.2011.01723.x. PMC 3151325 . PMID 21651511.
- 1 2 Villate O, Ibarluzea N, Maortua H, de la Hoz AB, Rodriguez-Revenga L, Izquierdo-Álvarez S, Tejada MI (2020-07-14). "Effect of AGG Interruptions on FMR1 Maternal Transmissions". Frontiers in Molecular Biosciences. 7: 135. doi: 10.3389/fmolb.2020.00135 . PMC 7381193 . PMID 32766278.
- ↑ Tabolacci E, Nobile V, Pucci C, Chiurazzi P (May 2022). "Mechanisms of the FMR1 Repeat Instability: How Does the CGG Sequence Expand?". International Journal of Molecular Sciences. 23 (10): 5425. doi: 10.3390/ijms23105425 . PMC 9141726 . PMID 35628235.
- ↑ "National Fragile X Foundation".
- ↑ "FRAXA".