
Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components:microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.
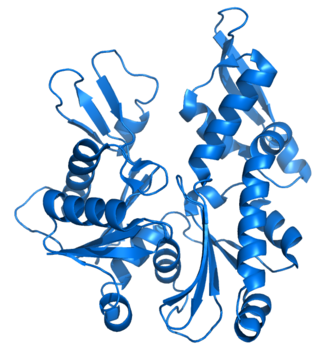
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin, found in eukaryotes, and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. It has been shown to form multilayer sheets comprising diagonally interwoven filaments in the presence of ATP or GTP.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

The nucleoid is an irregularly shaped region within the prokaryotic cell that contains all or most of the genetic material. The chromosome of a typical prokaryote is circular, and its length is very large compared to the cell dimensions, so it needs to be compacted in order to fit. In contrast to the nucleus of a eukaryotic cell, it is not surrounded by a nuclear membrane. Instead, the nucleoid forms by condensation and functional arrangement with the help of chromosomal architectural proteins and RNA molecules as well as DNA supercoiling. The length of a genome widely varies and a cell may contain multiple copies of it.

Filamentation is the anomalous growth of certain bacteria, such as Escherichia coli, in which cells continue to elongate but do not divide. The cells that result from elongation without division have multiple chromosomal copies.
Crescentin is a protein which is a bacterial relative of the intermediate filaments found in eukaryotic cells. Just as tubulins and actins, the other major cytoskeletal proteins, have prokaryotic homologs in, respectively, the FtsZ and MreB proteins, intermediate filaments are linked to the crescentin protein. Some of its homologs are erroneously labelled Chromosome segregation protein ParA. This protein family is found in Caulobacter and Methylobacterium.
The bacterium, despite its simplicity, contains a well-developed cell structure which is responsible for some of its unique biological structures and pathogenicity. Many structural features are unique to bacteria and are not found among archaea or eukaryotes. Because of the simplicity of bacteria relative to larger organisms and the ease with which they can be manipulated experimentally, the cell structure of bacteria has been well studied, revealing many biochemical principles that have been subsequently applied to other organisms.
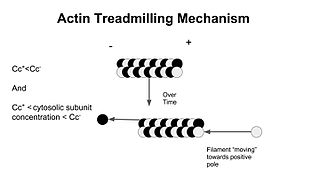
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling.
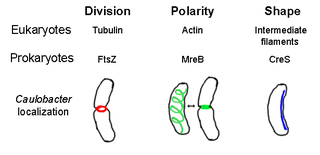
The prokaryotic cytoskeleton is the collective name for all structural filaments in prokaryotes. It was once thought that prokaryotic cells did not possess cytoskeletons, but advances in visualization technology and structure determination led to the discovery of filaments in these cells in the early 1990s. Not only have analogues for all major cytoskeletal proteins in eukaryotes been found in prokaryotes, cytoskeletal proteins with no known eukaryotic homologues have also been discovered. Cytoskeletal elements play essential roles in cell division, protection, shape determination, and polarity determination in various prokaryotes.
Fission, in biology, is the division of a single entity into two or more parts and the regeneration of those parts to separate entities resembling the original. The object experiencing fission is usually a cell, but the term may also refer to how organisms, bodies, populations, or species split into discrete parts. The fission may be binary fission, in which a single organism produces two parts, or multiple fission, in which a single entity produces multiple parts.
Bacterial morphological plasticity refers to changes in the shape and size that bacterial cells undergo when they encounter stressful environments. Although bacteria have evolved complex molecular strategies to maintain their shape, many are able to alter their shape as a survival strategy in response to protist predators, antibiotics, the immune response, and other threats.

Tubulin/FtsZ family, GTPase domain is an evolutionary conserved protein domain.

The Min System is a mechanism composed of three proteins MinC, MinD, and MinE used by E. coli as a means of properly localizing the septum prior to cell division. Each component participates in generating a dynamic oscillation of FtsZ protein inhibition between the two bacterial poles to precisely specify the mid-zone of the cell, allowing the cell to accurately divide in two. This system is known to function in conjunction with a second negative regulatory system, the nucleoid occlusion system (NO), to ensure proper spatial and temporal regulation of chromosomal segregation and division.
The MinC protein is one of three proteins in the Min system encoded by the minB operon and which is required to generate pole to pole oscillations prior to bacterial cell division as a means of specifying the midzone of the cell. This function is achieved by preventing the formation of the divisome Z-ring around the poles.
The MinE protein is one of three proteins of the Min system encoded by the minB operon required to generate pole to pole oscillations prior to bacterial cell division as a means of specifying the midzone of the cell, as seen in E.coli.
A plasmid partition system is a mechanism that ensures the stable inheritance of plasmids during bacterial cell division. Each plasmid has its independent replication system which controls the number of copies of the plasmid in a cell. The higher the copy number, the more likely the two daughter cells will contain the plasmid. Generally, each molecule of plasmid diffuses randomly, so the probability of having a plasmid-less daughter cell is 21−N, where N is the number of copies. For instance, if there are 2 copies of a plasmid in a cell, there is 50% chance of having one plasmid-less daughter cell. However, high-copy number plasmids have a cost for the hosting cell. This metabolic burden is lower for low-copy plasmids, but those have a higher probability of plasmid loss after a few generations. To control vertical transmission of plasmids, in addition to controlled-replication systems, bacterial plasmids use different maintenance strategies, such as multimer resolution systems, post-segregational killing systems, and partition systems.

FtsA is a bacterial protein that is related to actin by overall structural similarity and in its ATP binding pocket.

The divisome is a protein complex in bacteria that is responsible for cell division, constriction of inner and outer membranes during division, and peptidoglycan (PG) synthesis at the division site. The divisome is a membrane protein complex with proteins on both sides of the cytoplasmic membrane. In gram-negative cells it is located in the inner membrane. The divisome is nearly ubiquitous in bacteria although its composition may vary between species.
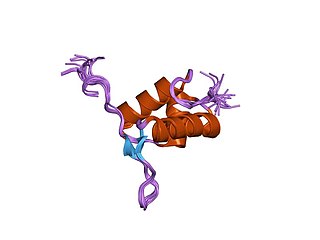
FtsK is a protein in E.Coli involved in bacterial cell division and chromosome segregation. It is one of the largest proteins, consisting of 1329 amino acids. FtsK stands for "Filament temperature sensitive mutant K" because cells expressing a mutant ftsK allele called ftsK44, which encodes an FtsK variant containing an G80A residue change in the second transmembrane segment, fail to divide at high temperatures and form long filaments instead. FtsK, specifically its C-terminal domain, functions as a DNA translocase, interacts with other cell division proteins, and regulates Xer-mediated recombination. FtsK belongs to the AAA superfamily and is present in most bacteria.















