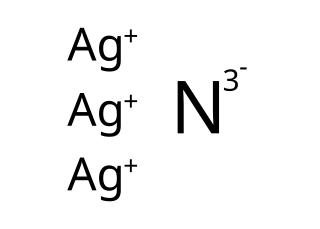Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.
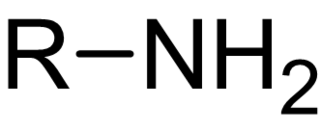
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.

Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but it turns yellow, orange or red within seconds from the formation of nitrosyl chloride and nitrogen dioxide. It was named by alchemists because it can dissolve noble metals like gold and platinum, though not all metals.

The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

Ammonium chloride is an inorganic chemical compound with the chemical formula NH4Cl, also written as [NH4]Cl. It is an ammonium salt of hydrogen chloride. It consists of ammonium cations [NH4]+ and chloride anions Cl−. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac. The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents. It is mainly used as fertilizer and a flavouring agent in some types of liquorice. It is a product of the reaction of hydrochloric acid and ammonia.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.
The nitrophosphate process is a method for the industrial production of nitrogen fertilizers invented by Erling Johnson in the municipality of Odda, Norway around 1927.

Nitrogen trichloride, also known as trichloramine, is the chemical compound with the formula NCl3. This yellow, oily, pungent-smelling and explosive liquid is most commonly encountered as a byproduct of chemical reactions between ammonia-derivatives and chlorine. Alongside monochloramine and dichloramine, trichloramine is responsible for the distinctive 'chlorine smell' associated with swimming pools, where the compound is readily formed as a product from hypochlorous acid reacting with ammonia and other nitrogenous substances in the water, such as urea from urine.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Ammonium nitrite, [NH4]NO2, is the ammonium salt of nitrous acid. It is not used in pure isolated form since it is highly unstable and decomposes into water and nitrogen, even at room temperature.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
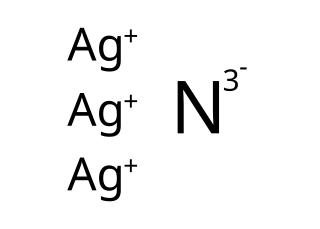
Silver nitride is an explosive chemical compound with symbol Ag3N. It is a black, metallic-looking solid which is formed when silver oxide or silver nitrate is dissolved in concentrated solutions of ammonia, causing formation of the diammine silver complex which subsequently breaks down to Ag3N. The standard free energy of the compound is about +315 kJ/mol, making it an endothermic compound which decomposes explosively to metallic silver and nitrogen gas.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.

Tris(trimethylsilyl)amine is the simplest tris(trialkylsilyl)amine which are having the general formula (R3Si)3N, in which all three hydrogen atoms of the ammonia are replaced by trimethylsilyl groups (-Si(CH3)3). Tris(trimethylsilyl)amine has been for years in the center of scientific interest as a stable intermediate in chemical nitrogen fixation (i. e. the conversion of atmospheric nitrogen N2 into organic substrates under normal conditions).